Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of a mono atomic gas of molar mass M undergoes a cyclic proce...
Text Solution
|
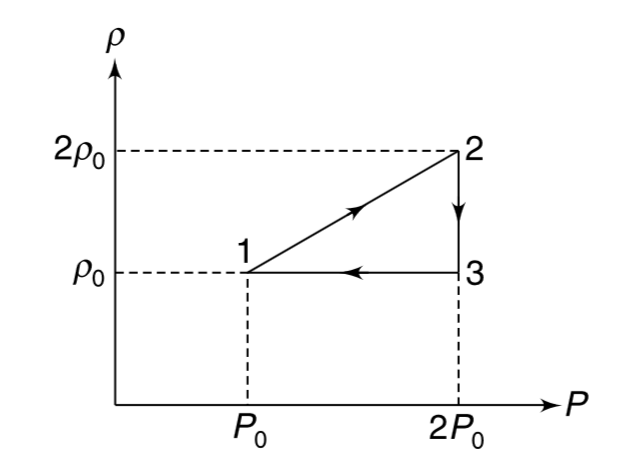
- The density (rho) versus pressure (p) graph of one mole of an ideal mo...
Text Solution
|
- Two moles of a monatomic ideal gas undergo a cyclic process ABCDA as s...
Text Solution
|
- One mole of an ideal mono-atomic gas is taken round cyclic process ABC...
Text Solution
|
- A monatomic ideal gas undergoes the shown cyclic process in which path...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a process as shown in th...
Text Solution
|
- One mole of an ideal gas is taken through the cyclic process ABCDA, as...
Text Solution
|
- n' moles of an ideal gas having molar mass M is made to undergo a cycl...
Text Solution
|
- One mole of a mono atomic gas of molar mass M undergoes a cyclic proce...
Text Solution
|