Similar Questions
Explore conceptually related problems
Recommended Questions
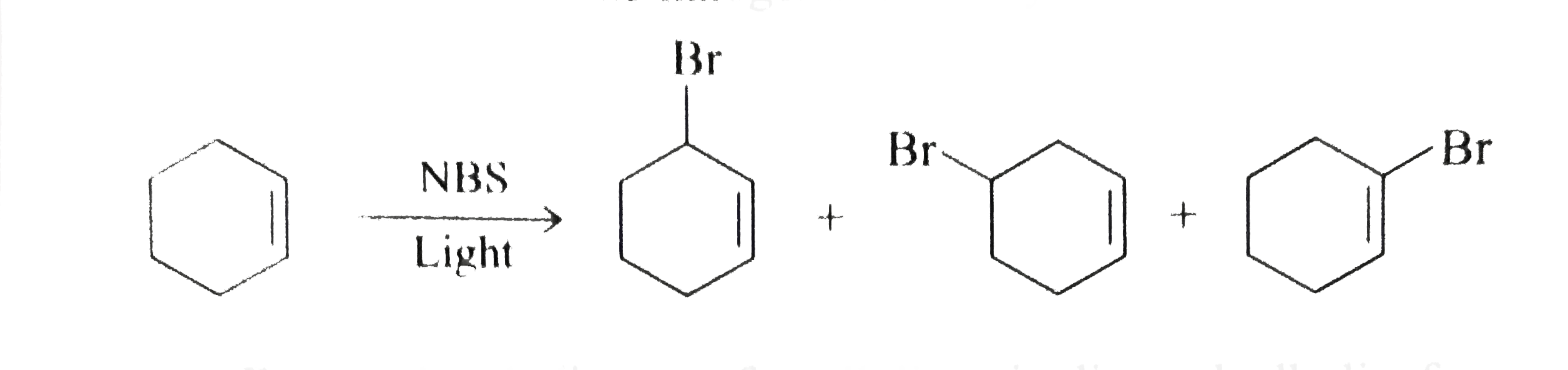
- Karl Ziegler reported that alkenes react with N-bromosuccinimide (NBS)...
Text Solution
|
- Assertion: NBS is a specific reagent for allylic bromination Reason: A...
Text Solution
|
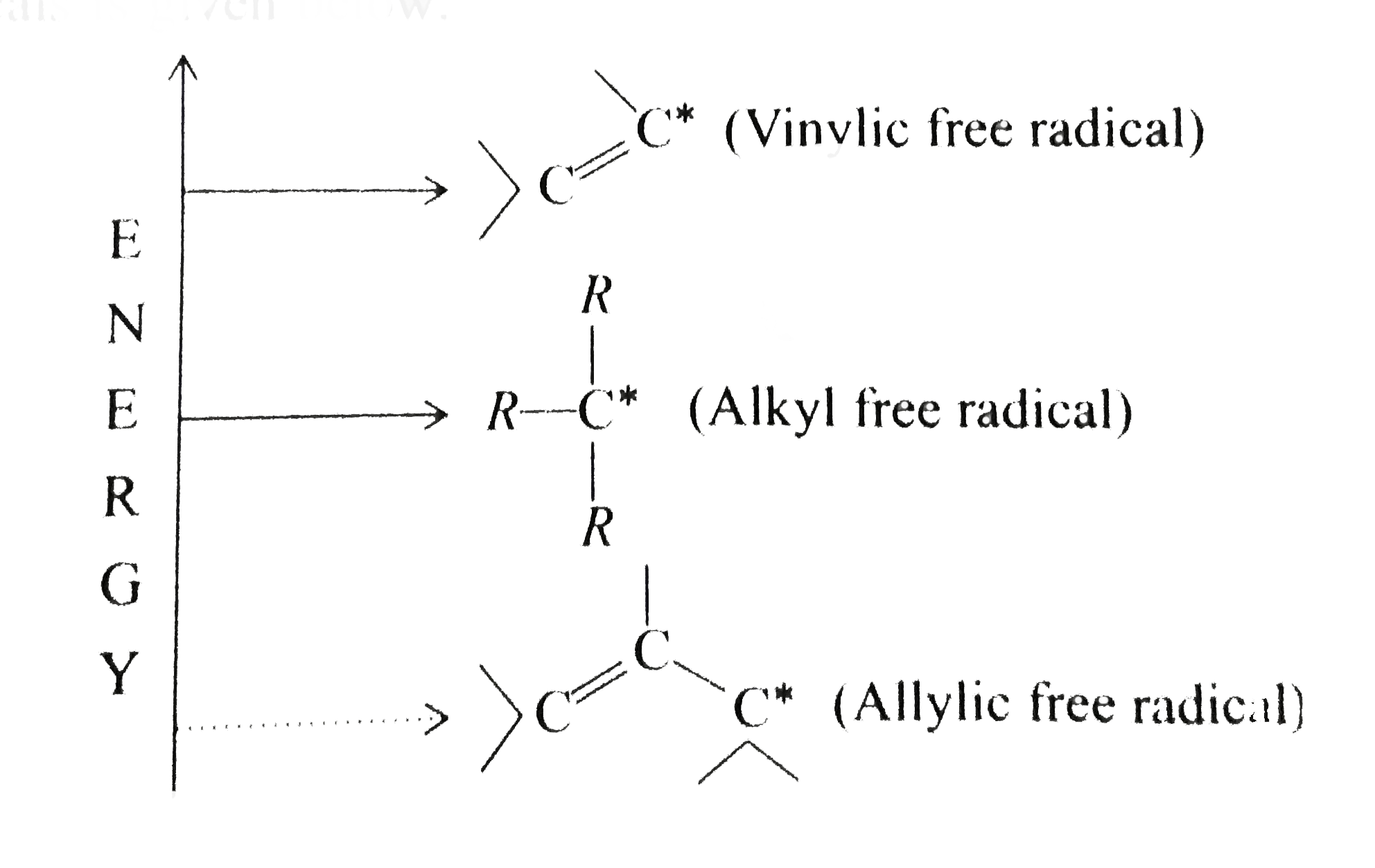
- Consider the molecule shown below and of stability (1=moststable ) ...
Text Solution
|
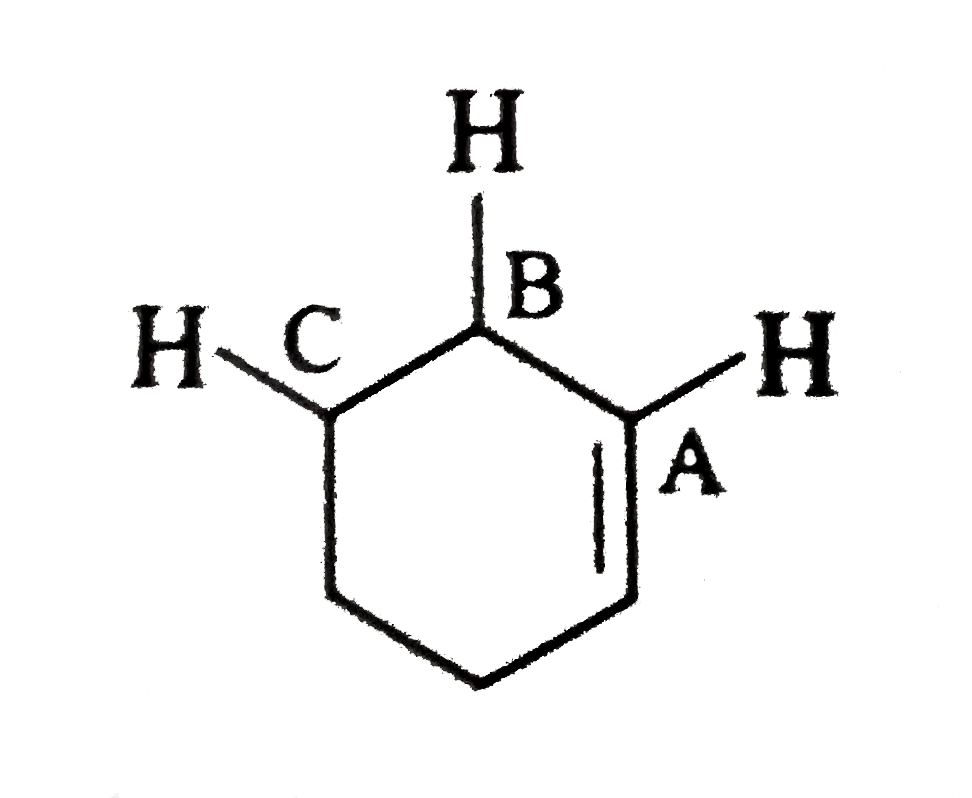
- Indicate allylic, vinyl, 1^(@), 2^(@),3^(@) hydrogen in the following ...
Text Solution
|
- Karl Ziegler reported that alkenes react with N-bromosuccinimide (NBS)...
Text Solution
|
- Karl Ziegler reported that alkenes react with N-bromosuccinimide (NBS)...
Text Solution
|
- Karl Ziegler reported that alkenes react with N-bromosuccinimide (NBS)...
Text Solution
|
- Karl Ziegler reported that alkenes react with N-bromosuccinimide (NBS)...
Text Solution
|
- (A) In allylic substitution propene gives allyl bromide. (R) NBS is ...
Text Solution
|