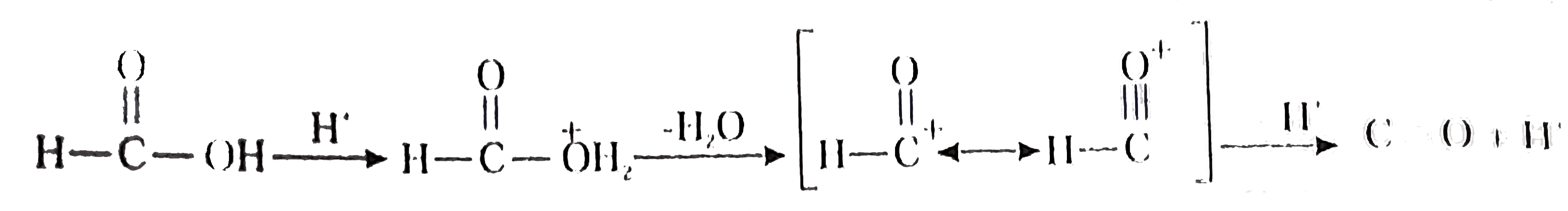Similar Questions
Explore conceptually related problems
Recommended Questions
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Oxalic acid when heated with conc. H2 SO4 it gives out
Text Solution
|
- Formic acid when heated with conc . H2 SO4 produces …….. .
Text Solution
|
- The reaction of formic acid with concentrated H2 SO4 gives
Text Solution
|
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Methanoic acid, the first member of carboxylic acid series, when warme...
Text Solution
|
- Formic acid is not a representative member of the carboxylic acids bec...
Text Solution
|