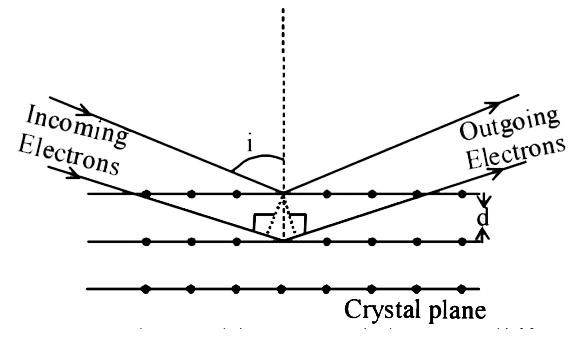
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PHOTONS AND MATTER WAVES
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS(Matrix - Match)|5 VideosPHOTONS AND MATTER WAVES
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS(Integer Type)|4 VideosPHOTONS AND MATTER WAVES
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (More than One Correct Choice Type)|5 VideosOSCILLATIONS
RESNICK AND HALLIDAY|Exercise Practice Questions|57 VideosRELATIVITY
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (Integer Type)|5 Videos
Similar Questions
Explore conceptually related problems
RESNICK AND HALLIDAY-PHOTONS AND MATTER WAVES-PRACTICE QUESTIONS (Link Comprehension)
- which of the following are not dependent on the intensity of the incid...
Text Solution
|
- It is desired to obtain a diffraction pattern for electrons using a di...
Text Solution
|
- It is desired to obtain a diffraction pattern for electrons using a di...
Text Solution
|
- A physicist wishes to eject electrons by shining light on a metal surf...
Text Solution
|
- A physicist wishes to eject electrons by shining light on a metal surf...
Text Solution
|
- When a particle is restricted to move aong xaxis between x =0 and x = ...
Text Solution
|
- When a particle is restricted to move aong xaxis between x =0 and x = ...
Text Solution
|
- Wave property of electron implies that they will show diffraction effe...
Text Solution
|
- Wave property of electron implies that they will show diffraction effe...
Text Solution
|
- Photoelectrons ar ejected from a surface when light of wavelenght lamd...
Text Solution
|
- Photoelectrons ar ejected from a surface when light of wavelenght lamd...
Text Solution
|
- Photoelectrons ar ejected from a surface when light of wavelenght lamd...
Text Solution
|
- When a surface is irradiated with light of wavelength 4950 Å, a photo...
Text Solution
|
- When a surface is irradiated with light of wavelength 4950 Å, a photo...
Text Solution
|
- When a surface is irradiated with a light of wavelength 4950 Å, a phot...
Text Solution
|
- In Young’s double slit experiment an electron beam is used to form a f...
Text Solution
|
- A beam of electrons with speed v(0) passes through double slits and th...
Text Solution
|