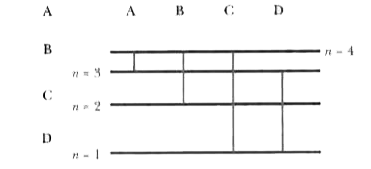
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN ATOM
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (More than One Correct Choice Type)|15 VideosHYDROGEN ATOM
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (Linked Comprehension)|9 VideosHYDROGEN ATOM
RESNICK AND HALLIDAY|Exercise PROBLEMS|36 VideosHEAT-MEASUREMENT AND TRANSFER
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS( INTEGER TYPE)|5 VideosINTERFERENCE AND DIFFRACTION
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (Integer Type)|6 Videos
Similar Questions
Explore conceptually related problems
RESNICK AND HALLIDAY-HYDROGEN ATOM-PRACTICE QUESTIONS (Single Correct Choice Type)
- If the shortest wavelength of Lyman series of hydrogen atom is x, then...
Text Solution
|
- A particle in a box has quantum states with energies E= E(0) n^(2), wi...
Text Solution
|
- Which of the following transitions produce the longest wavelength phot...
Text Solution
|
- For a quantum particle in a box, the lowest energy quantum state has 1...
Text Solution
|
- An electron is in a one-dimensional trap with zero potential energy in...
Text Solution
|
- A particle is trapped in an infinite potential energy well. It is in t...
Text Solution
|
- A particle is confined to a one-dimensional trap by infinite potential...
Text Solution
|
- In the Bohr model of hydrogen, why is the atom 9 times larger in the n...
Text Solution
|
- Orbital electrons do not spiral into the nucleus because of
Text Solution
|
- Bohr's model cannot explain the spectrum of neutral Lithium atoms beca...
Text Solution
|
- Compared to hydrogen the atom of helium has
Text Solution
|
- If the value of Planck's constant were to increase by a factor of two,...
Text Solution
|
- In the Bohr model, if an electron moves in an orbit of greater radius
Text Solution
|
- Which of the following statements is (are) wrong for hydrogen atom?
Text Solution
|
- The total energy of an electron in the excited state corresponding to ...
Text Solution
|
- Identify the highest energy photons of the following
Text Solution
|
- An atom consists of theree energy levels given by a ground state with ...
Text Solution
|
- When radiation with a continuous spectrum is passed through a volume o...
Text Solution
|
- Which of the following statement is correct in connection with hydroge...
Text Solution
|
- A hydrogen atom is excited up to 9th level. The total number of possib...
Text Solution
|