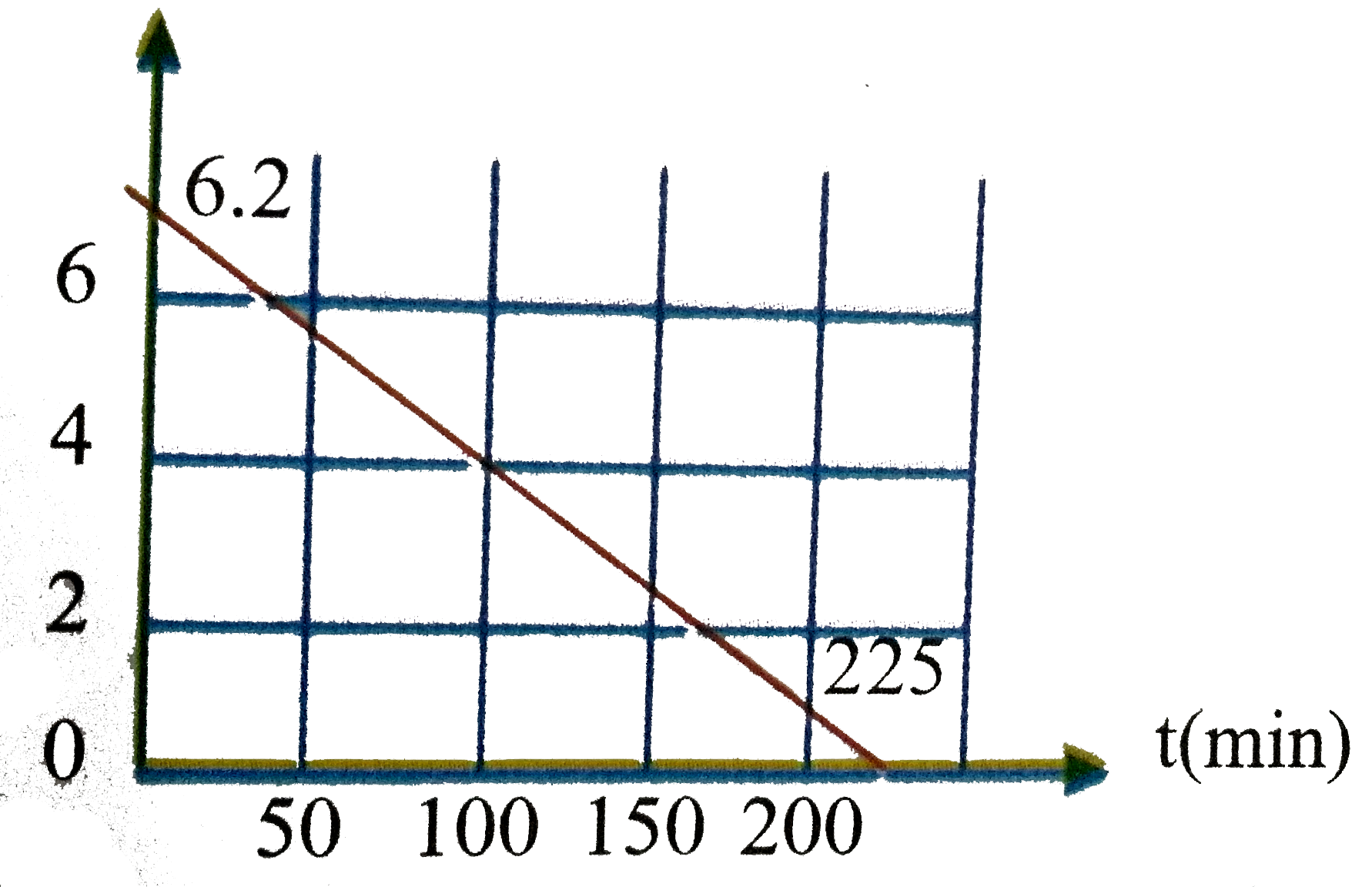
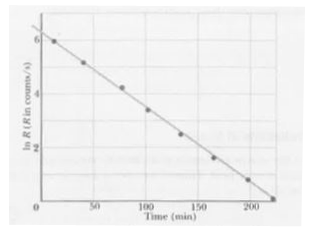
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESNICK AND HALLIDAY-THE NUCLEUS-PRACTICE QUESTIONS(Integer Type)
- The table that follows shows some measurements of the decay rate of a ...
Text Solution
|
- A neutron with kinetic energy K=10MeV activates an endoergic nuclear r...
Text Solution
|
- Two radioactive substances X and Y initially contain an equal number o...
Text Solution
|
- There are two radioactive substance A and B. Decay consant of B is two...
Text Solution
|
- In the final Uranium radioactive series the initial nucleus is U(92)^(...
Text Solution
|
- A freshly prepared sample of a radioisotope of half - life 1386 s has...
Text Solution
|
- A nuclear power supplying electrical power to a villages uses a radioa...
Text Solution
|
- ""^(131)I is an isotope of Iodine that beta- decays to an isotope of x...
Text Solution
|