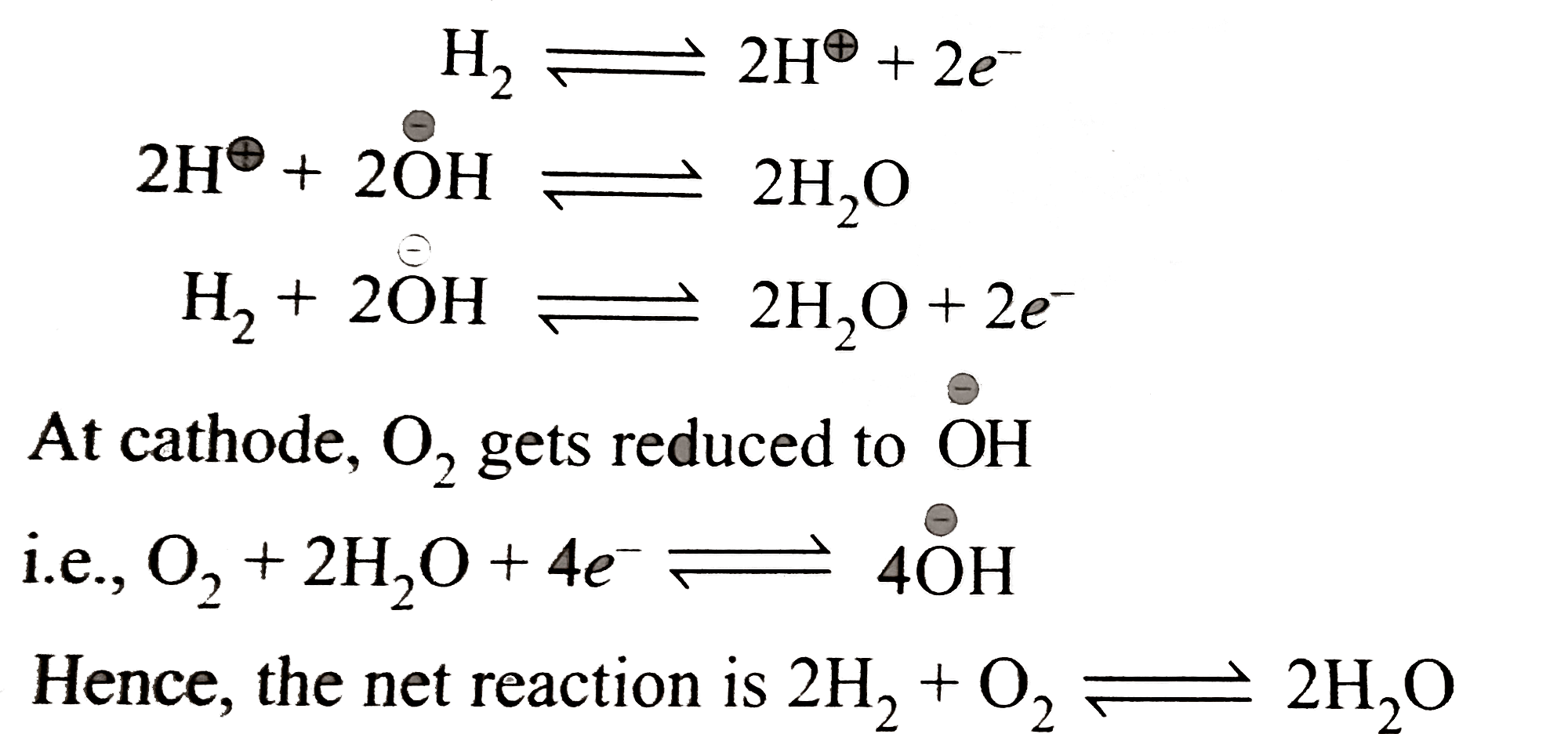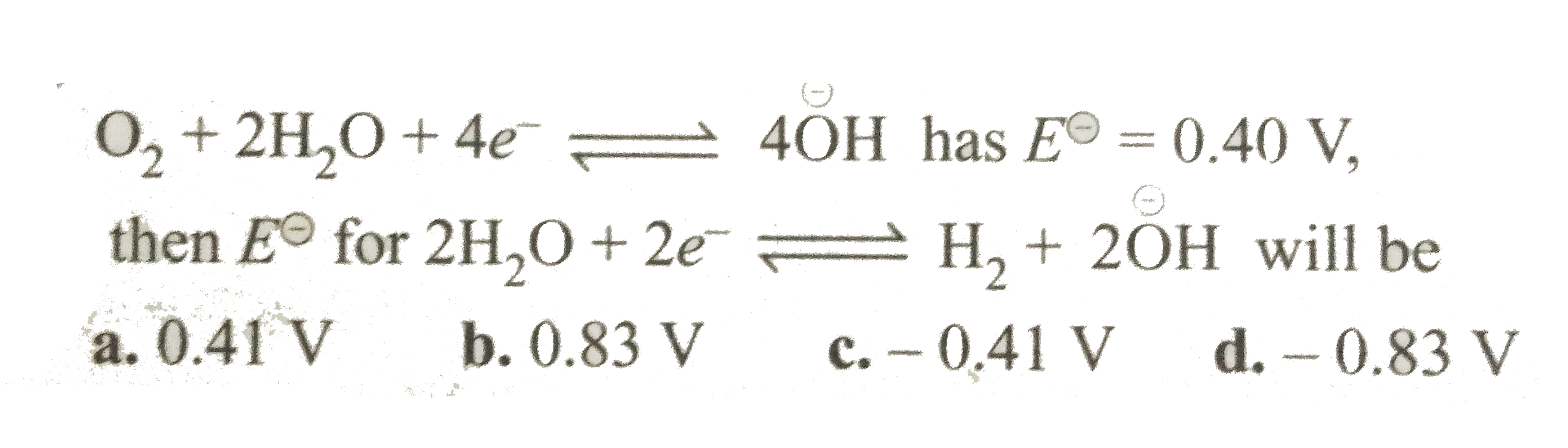A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (OBJECTIVE TYPE QUESTIONS) MATRIX MATCH TYPE QUESTIONS|3 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (OBJECTIVE TYPE QUESTIONS)Matching Type Questions|1 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (OBJECTIVE TYPE QUESTIONS) C MULTIPLE CHOICE QUESTIONS|10 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|12 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MODERN PUBLICATION|Exercise COMPETION FILE (Integer Type Numerical Value Type Questions)|5 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ELECTROCHEMISTRY -COMPETITION FILE (OBJECTIVE TYPE QUESTIONS) D MULTIPLE CHOICE QUESTIONS
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- The concentration of potassium ions inside a biological cell is at le...
Text Solution
|
- The concentration of potassium ions inside a biological cell is at lea...
Text Solution
|
- The electrochemical cell shown below is a concentration cell M//M^(...
Text Solution
|
- The electrochemical cell shown below is a concentration cell. M|M^(2+)...
Text Solution
|