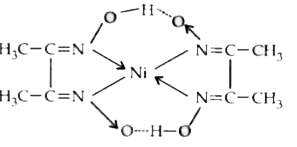
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
MTG-WBJEE|Exercise WB JEE Previous Years Questons (Category 1 : Single Option Correct Type )|9 VideosATOMS MOLECULES AND CHEMICAL ARITHEMETIC
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2: SINGLE OPTION CORRECT TYPE (2 MARKS))|3 VideosCHEMICAL DYNAMICS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 3 : One or More than One Option Correct Type)(2 Marks)|2 Videos
Similar Questions
Explore conceptually related problems