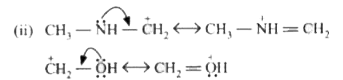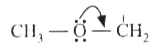A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMISTRY OF CARBON COMPOUNDS
MTG-WBJEE|Exercise WB JEE/ WORKOUT (CATEGORY 3 : ONE OR MORE THAN ONE OPTION CORRECT TYPE )|10 VideosCHEMISTRY OF CARBON COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 1 : SINGLE OPTION CORRECT TYPE )|17 VideosCHEMISTRY OF CARBON COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 3 : ONE OR MORE THAN ONE OPTION CORRECT TYPE )|3 VideosCHEMISTRY IN INDUSTRY
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (SINGLE OPTION CORRECT TYPE)|1 VideosCHEMISTRY OF METALS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)|5 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-CHEMISTRY OF CARBON COMPOUNDS -WB JEE/ WORKOUT (CATEGORY 2 : SINGLE OPTION CORRECT TYPE )
- A compound is formed by substitution of two chlorine for two hydrogens...
Text Solution
|
- The most contributing tautomeric enol form of "MecCOCH"(2)CO(2) Et is
Text Solution
|
- Among the following carbocations : (I) Ph(2) C^(+) CH(2) Me (II) P...
Text Solution
|
- The total number of acyclic and cyclic isomers including geometrical i...
Text Solution
|
- Which of the following statements are true with respect to electronic ...
Text Solution
|
- In which of the following pairs of carbocations, the first carbocation...
Text Solution
|
- Tautomerism is not exhibited by
Text Solution
|
- Two isomeric alkenes A and B having molecular formula, CH(9)H(9) Cl o...
Text Solution
|
- The number of chiral carbon atoms in I, II , III respectively are I...
Text Solution
|
- An optically active alkene with the molecular fomula C(6) CH(12) which...
Text Solution
|
- The number of stereoisomers and optical isomers possible for the compo...
Text Solution
|
- Which of the following molecules will not show optical activity?
Text Solution
|
- Select the S-isomer from the following:
Text Solution
|
- Consider the following conversions : I. CH(3) underset(CN)underset(|...
Text Solution
|
- Given below are the structures of five organic compounds (1) to (5) wh...
Text Solution
|