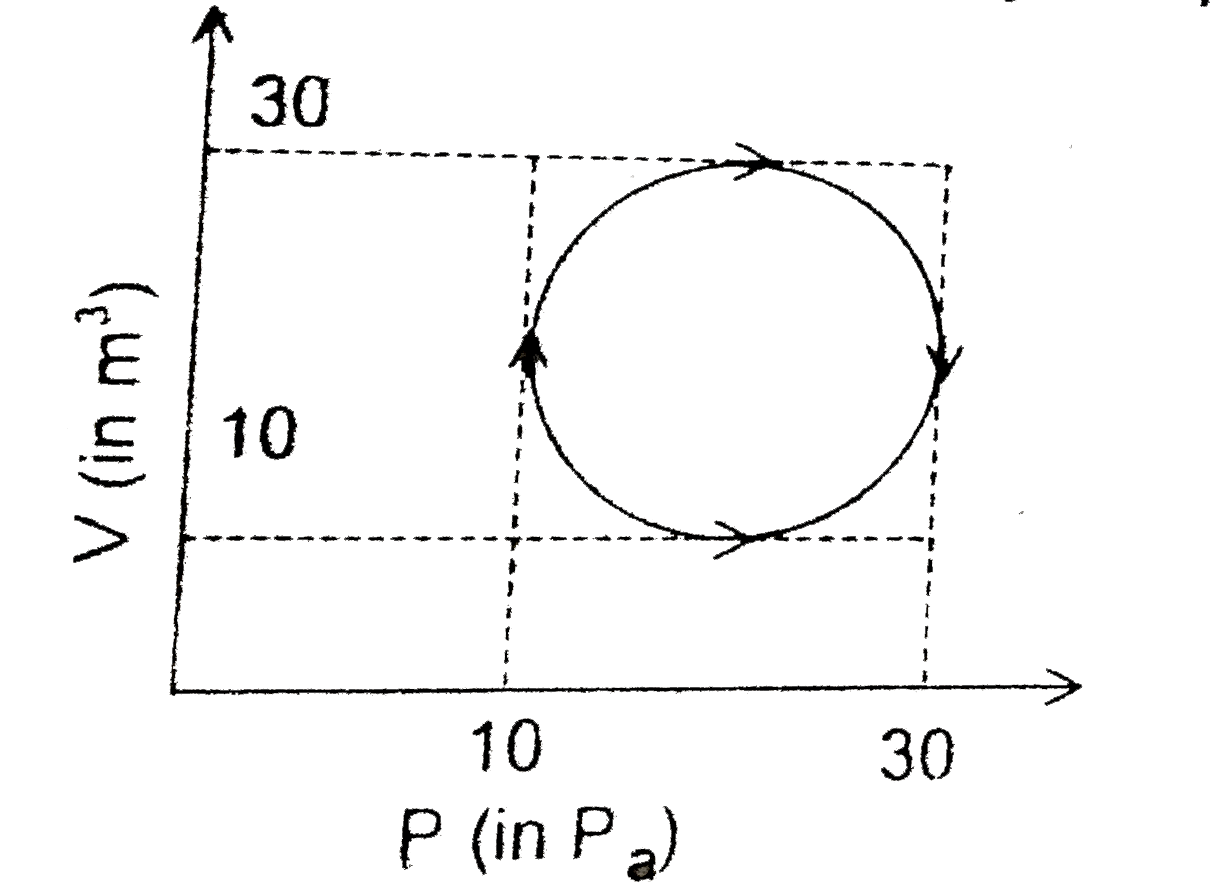Similar Questions
Explore conceptually related problems
Recommended Questions
- A system works under cyclic process as follow Heat absorbed during t...
Text Solution
|
- Heat energy absorbed by a system in going through a cyclic process sho...
Text Solution
|
- Heat energy absorbed by a system in going through a cyclic process sho...
Text Solution
|
- A system works under cyclic process as follow Heat absorbed during t...
Text Solution
|
- The heat absorbed by the system in going through the cyclic process as...
Text Solution
|
- A system works in a cyclic process. It absorbs 20 calories of heat and...
Text Solution
|
- What would be the heat absorbed by the system in the cyclic process?
Text Solution
|
- A : During a cyclic process work done by the system is zero. R : Heat ...
Text Solution
|
- A system undergoes a cyclic process in which it absorbs heat Q1 and gi...
Text Solution
|