Assertion: `HCHO` is always oxidized in the crossed Cannizzaro reaction. Reason : `HCHO` is the most reactive aldehyde, it axist in aqueous `OH^(-)` solution as the conjugate base of its hydrate
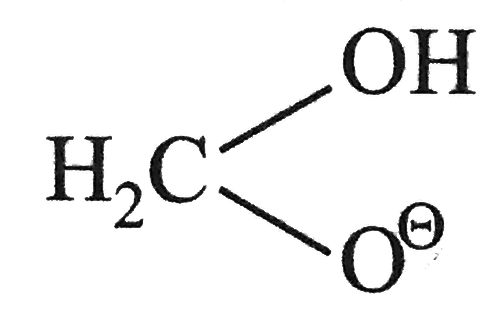 , there is also a statistical factor because `HCHO` has two aldehydic hydrogen available for transfer whilein other aldehyde hydrate anion has only one such hydrogen atom.
, there is also a statistical factor because `HCHO` has two aldehydic hydrogen available for transfer whilein other aldehyde hydrate anion has only one such hydrogen atom.
Assertion: `HCHO` is always oxidized in the crossed Cannizzaro reaction. Reason : `HCHO` is the most reactive aldehyde, it axist in aqueous `OH^(-)` solution as the conjugate base of its hydrate
 , there is also a statistical factor because `HCHO` has two aldehydic hydrogen available for transfer whilein other aldehyde hydrate anion has only one such hydrogen atom.
, there is also a statistical factor because `HCHO` has two aldehydic hydrogen available for transfer whilein other aldehyde hydrate anion has only one such hydrogen atom.
 , there is also a statistical factor because `HCHO` has two aldehydic hydrogen available for transfer whilein other aldehyde hydrate anion has only one such hydrogen atom.
, there is also a statistical factor because `HCHO` has two aldehydic hydrogen available for transfer whilein other aldehyde hydrate anion has only one such hydrogen atom.Similar Questions
Explore conceptually related problems
The conversion of aldehyde having no alpha hydrogen to a mixture to a mixture of carboxylic acid and primary alcohol is known as cannizzaro reaction. The most important feature of this reaction is the conjugates base of hydrate aldehyde. In the given reaction final product is
The conversion of aldehyde having no alpha hydrogen to a mixture to a mixture of carboxylic acid and primary alcohol is known as cannizzaro reaction. The most important feature of this reaction is the conjugates base of hydrate aldehyde. Which step is rate determine step
The conversion of aldehyde having no alpha hydrogen to a mixture to a mixture of carboxylic acid and primary alcohol is known as cannizzaro reaction. The most important feature of this reaction is the conjugates base of hydrate aldehyde. Which step is rate determine step
Read the passage given below and answer the following questions: Reductive alkylation is the term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of an aldehyde or ketone in the presence of a reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or "nascent" hydrogen, usually from a metalacid combination, most of these reductive alkylations have been carried out with hydrogen and a catalyst. The principal variation excluded is that in which the reducing agent is formic acid or one of its derivatives, this modification is known as the Leuckart reaction. The process of reductive alkylation of ammonia consists in the addition of ammonia to a carbonyl compound and reduction of the addition compound or its dehydration product. The reaction usually is carried out in ethanol solution when the reduction is to be effected catalytically Since the primary amine is formed in the presence of the aldehyde it may react in the same way as ammonia, yielding an addition compound, a Schiff's base (RCH= NCH_(2)R) and finally, a secondary amine. Similarly, the primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines. Similar reactions may occur when the carbonyl compound employed is a ketone. (source: Emerson, W. S. (2011). The Preparation of Amines by Reductive Alkylation. Organic Reactions, 174–255. doi:10.1002/0471264180.or004.03 ) Reductive alkylation of ammonia by means of an aldehyde in presence of hydrogen as reducing agents results in formation of:
Read the passage given below and answer the following questions: Reductive alkylation is the term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of an aldehyde or ketone in the presence of a reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or "nascent" hydrogen, usually from a metalacid combination, most of these reductive alkylations have been carried out with hydrogen and a catalyst. The principal variation excluded is that in which the reducing agent is formic acid or one of its derivatives, this modification is known as the Leuckart reaction. The process of reductive alkylation of ammonia consists in the addition of ammonia to a carbonyl compound and reduction of the addition compound or its dehydration product. The reaction usually is carried out in ethanol solution when the reduction is to be effected catalytically Since the primary amine is formed in the presence of the aldehyde it may react in the same way as ammonia, yielding an addition compound, a Schiff's base (RCH= NCH_(2)R) and finally, a secondary amine. Similarly, the primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines. Similar reactions may occur when the carbonyl compound employed is a ketone. (source: Emerson, W. S. (2011). The Preparation of Amines by Reductive Alkylation. Organic Reactions, 174–255. doi:10.1002/0471264180.or004.03 ) The reaction of ammonia and its derivatives with aldehydes is called:
Read the passage given below and answer the following questions: Reductive alkylation is the term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of an aldehyde or ketone in the presence of a reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or "nascent" hydrogen, usually from a metalacid combination, most of these reductive alkylations have been carried out with hydrogen and a catalyst. The principal variation excluded is that in which the reducing agent is formic acid or one of its derivatives, this modification is known as the Leuckart reaction. The process of reductive alkylation of ammonia consists in the addition of ammonia to a carbonyl compound and reduction of the addition compound or its dehydration product. The reaction usually is carried out in ethanol solution when the reduction is to be effected catalytically Since the primary amine is formed in the presence of the aldehyde it may react in the same way as ammonia, yielding an addition compound, a Schiff's base (RCH= NCH_(2)R) and finally, a secondary amine. Similarly, the primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines. Similar reactions may occur when the carbonyl compound employed is a ketone. (source: Emerson, W. S. (2011). The Preparation of Amines by Reductive Alkylation. Organic Reactions, 174–255. doi:10.1002/0471264180.or004.03 ) Acetaldehyde is reacted with ammonia followed by reduction in presence of hydrogen as a catalyst. The primary amine so formed further reacts with acetaldehyde. The Schiff’s base formed during the reaction is:
Read the passage given below and answer the following questions: Reductive alkylation is the term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of an aldehyde or ketone in the presence of a reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or "nascent" hydrogen, usually from a metalacid combination, most of these reductive alkylations have been carried out with hydrogen and a catalyst. The principal variation excluded is that in which the reducing agent is formic acid or one of its derivatives, this modification is known as the Leuckart reaction. The process of reductive alkylation of ammonia consists in the addition of ammonia to a carbonyl compound and reduction of the addition compound or its dehydration product. The reaction usually is carried out in ethanol solution when the reduction is to be effected catalytically Since the primary amine is formed in the presence of the aldehyde it may react in the same way as ammonia, yielding an addition compound, a Schiff's base (RCH= NCH_(2)R) and finally, a secondary amine. Similarly, the primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines. Similar reactions may occur when the carbonyl compound employed is a ketone. (source: Emerson, W. S. (2011). The Preparation of Amines by Reductive Alkylation. Organic Reactions, 174–255. doi:10.1002/0471264180.or004.03 ) Ethanal on reaction with ammonia forms an imine (X) which on reaction with nascent hydrogen gives (Y). Identify ‘X’ and ‘Y’.
Recommended Questions
- Assertion: HCHO is always oxidized in the crossed Cannizzaro reaction...
Text Solution
|
- Assertion (A): The following cross Cannizzaro reaction occurs. Re...
Text Solution
|
- Assertion: HCHO is always oxidized in the crossed Cannizzaro reaction...
Text Solution
|
- Assertion : Aromatic aldehydes and formadehyde undergo Cannizzaro reac...
Text Solution
|
- The conversion of aldehyde having no alpha hydrogen to a mixture of ca...
Text Solution
|
- The conversion of aldehyde having no alpha hydrogen to a mixture of ca...
Text Solution
|
- The conversion of aldehyde having no alpha hydrogen to a mixture to a ...
Text Solution
|
- The conversion of aldehyde having no alpha hydrogen to a mixture to a ...
Text Solution
|
- HCHO को छोड़कर शेष सभी ऐल्डिहाइडों को रोजेनमुण्ड अपचयन द्वारा बनाया जा...
Text Solution
|