Similar Questions
Explore conceptually related problems
Recommended Questions
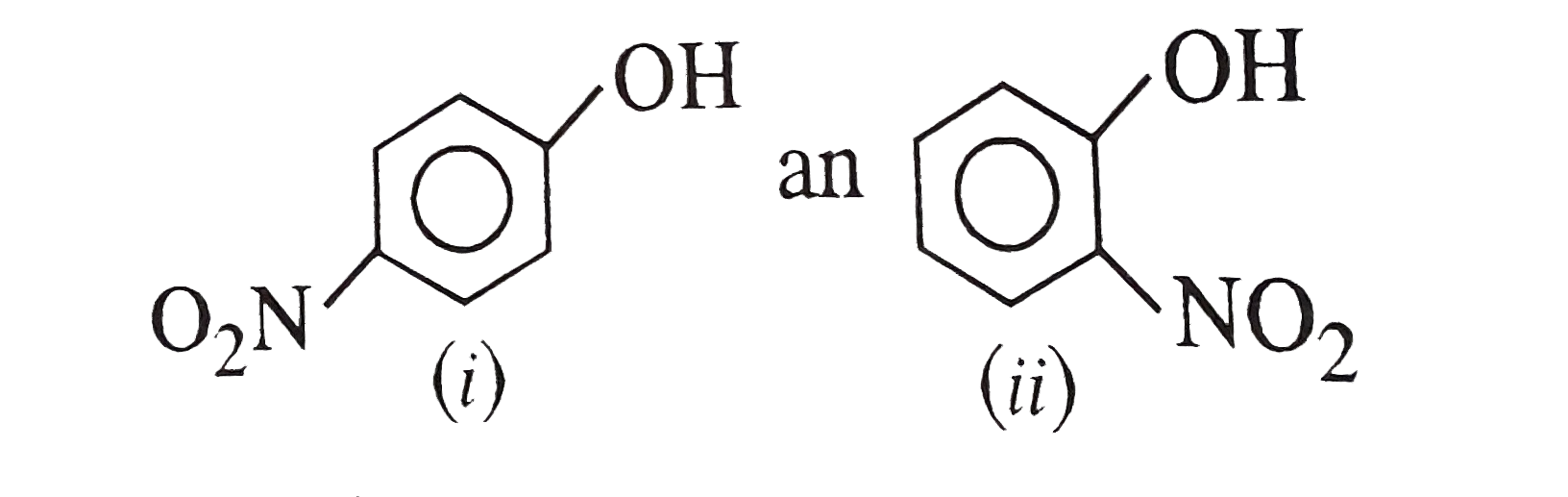
- For the two compounds, the vapour pressure of (i) at a particular temp...
Text Solution
|
- At a particular temperature why is the vapour pressure of acetone less...
Text Solution
|
- Out of the two compounds shown below the vapour pressure of II at a pa...
Text Solution
|
- Of the two compounds shown below , the vapour pressure of B at a parti...
Text Solution
|
- Consider the following compounds Among the compounds A and B, the vapo...
Text Solution
|
- For the two compounds, the vapour pressure of (i) at a particular temp...
Text Solution
|
- At a particular temperature why is the vapour pressure of acetone less...
Text Solution
|
- Of the two compounds shown below , the vapour pressure of B at a par...
Text Solution
|
- Out of the two compounds shown below, the vapour pressure of B at a pa...
Text Solution
|