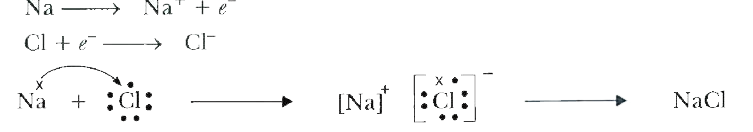Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
VK GLOBAL PUBLICATION|Exercise HOTS (HIGHER ORDER THINKING SKILLS)|6 VideosMETALS AND NON-METALS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE VERY SHORT ANSWER|5 VideosMETALS AND NON-METALS
VK GLOBAL PUBLICATION|Exercise SHORT ANSWER QUESTION -II|20 VideosCHEMICAL REACTIONS AND EQUATIONS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (LONG ANSWER QUESTIONS)|2 VideosMODEL QUESTION PAPER - 4
VK GLOBAL PUBLICATION|Exercise SECTION - B |2 Videos
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-METALS AND NON-METALS-LONG ANSWER QUESTIONS
- Give one example of 1315 compounds.
Text Solution
|
- Two ores A and B were taken. On heating, ore A gives CO(2) whereas, or...
Text Solution
|
- Write the names and symbols of two most reactive metals. Explain by dr...
Text Solution
|
- Hydrogen is not a metal but is has been assigned a place in the reacti...
Text Solution
|
- How would you show that silver is chemically less reactive than copper...
Text Solution
|
- What is an ionic bond?
Text Solution
|
- How is an ionic bond formed?
Text Solution
|
- Write the formation of magnesium chloride.
Text Solution
|
- Distinguish between ionic and covalent compounds under the following p...
Text Solution
|
- Explain how the following metals are obtained from their compound by t...
Text Solution
|
- Distinguish between roasting and calcination . Which of these two is u...
Text Solution
|
- Write a chemical equation to illustrate the use of aluminium for joini...
Text Solution
|
- Name the anode,the cathode and the electrolyte used in the electrolyti...
Text Solution
|
- Write about different chemical process used for obtaining a metal from...
Text Solution
|
- How do you classify elements into metals and non metals on the basis o...
Text Solution
|
- What type of bond will be formed if? (a) A combines with B? (b) A co...
Text Solution
|