Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON AND ITS COMPOUNDS
VK GLOBAL PUBLICATION|Exercise NCERT Exercises|15 VideosCARBON AND ITS COMPOUNDS
VK GLOBAL PUBLICATION|Exercise VERY SHORT ANSWER QUESTIONS|33 VideosACIDS, BASES AND SALTS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE ( Long Answer Questions (5 mark))|4 VideosCHEMICAL REACTIONS AND EQUATIONS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (LONG ANSWER QUESTIONS)|2 Videos
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-CARBON AND ITS COMPOUNDS-PROFICIENCY EXERCISE (Long Answer Questions)
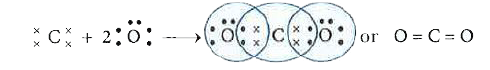
- What would be the electron dot structure of carbon dioxide which has t...
Text Solution
|
- What are hydrocarbons? Distinguish alkanes from alkenes and each of th...
Text Solution
|
- An organic compound 'A' on heating with another compound 'B' in presen...
Text Solution
|
- Explain the following term with the help of chemical reaction: (i) O...
Text Solution
|
- Name the functional groups present in the following compounds. (a) ...
Text Solution
|
- Match the reactions given in Column (A) with the names given in Column...
Text Solution
|
- What is meant by "structural isomers"? Give reason why propane (C(3)H(...
Text Solution
|