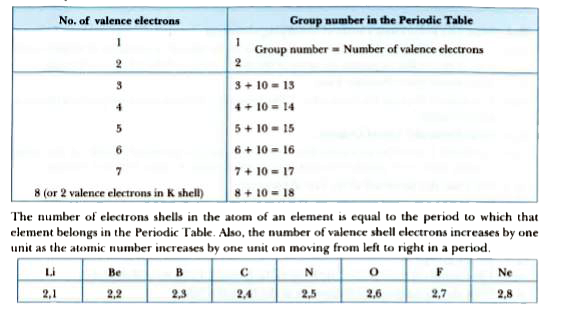
Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise VERY SHORT ANSWER QUESTIONS|14 VideosPERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise SHORT ANSWER QUESTIONS-I|23 VideosPERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (LONG ANSWER QUESTIONS)|3 VideosMODEL QUESTIONS PAPER - 5
VK GLOBAL PUBLICATION|Exercise SECTION B|3 VideosPRACTICAL BASED QUESTIONS
VK GLOBAL PUBLICATION|Exercise Experiment 15|4 Videos
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -NCERT EXERCISES
- Which of the following statements is not a correct statement about the...
Text Solution
|
- Element X forms a chloride with the formula XCl(2), which is a solid w...
Text Solution
|
- Which element has two shells, both of which are completely filled with...
Text Solution
|
- Which element has the electronic configuration 2, 8, 2?
Text Solution
|
- Which element has a total of three shells, with four electrons in its ...
Text Solution
|
- Which element has a total of two shells, with three electrons in its v...
Text Solution
|
- Which element has twice as many electrons in its second shell as in i...
Text Solution
|
- (a) What property do all elements in the same column of the Periodic T...
Text Solution
|
- An atom has electronic configuration 2, 8, 7. (a) What is the atomic n...
Text Solution
|
- The position of three elements A, B and C in the Periodic Table are sh...
Text Solution
|
- Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to...
Text Solution
|
- How does the electronic configuration of an atom relate to its positio...
Text Solution
|
- In the Modern Periodic Table, calcium (atomic number 20) is surrounded...
Text Solution
|
- Compare and contrast the arrangement of elements in Mendeléev’s Period...
Text Solution
|