Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise LONG ANSWER QUESTIONS|17 VideosPERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS|10 VideosPERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise SHORT ANSWER QUESTIONS-I|23 VideosMODEL QUESTIONS PAPER - 5
VK GLOBAL PUBLICATION|Exercise SECTION B|3 VideosPRACTICAL BASED QUESTIONS
VK GLOBAL PUBLICATION|Exercise Experiment 15|4 Videos
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -SHORT ANSWER QUESTIONS-II
- Write the number of periods the Modern Periodic Table has. State the c...
Text Solution
|
- The elements ""4Be, ""12Mg and ""20Ca , each having two valence electr...
Text Solution
|
- Given below are some elements of the Modern Periodic Table. Atomic num...
Text Solution
|
- Nitrogen (atomic no. 7) and phosphorous (atomic no. 15) belong to grou...
Text Solution
|
- Na, Mg and Al are the elements having one, two and three valence elect...
Text Solution
|
- Two elements 'P' and 'Q' belong to the same period of the modern perio...
Text Solution
|
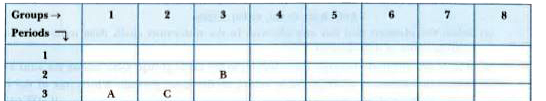
- The position of three elements A, B and C in the Periodic Table is sho...
Text Solution
|
- Identify, the elements with the following property and arrange them in...
Text Solution
|
- Properties of the elements are given below. Where would you locate the...
Text Solution
|
- The atomic number of an element “X' is 20. (i) Determine the posi...
Text Solution
|
- The following table shows the position of six elements A, B, C, D, E a...
Text Solution
|
- The atomic radii of first group elements of the Periodic Table are as ...
Text Solution
|
- The elements of the second period of the Periodic Table are given belo...
Text Solution
|
- The atomic numbers of nitrogen, oxygen and fluorine are 7, 8 and 9 res...
Text Solution
|
- Four elements P, Q, R and S belong to the third period of the Modern P...
Text Solution
|
- Consider the following elements: Li, Cl, Br, Na, K, I (i) Arran...
Text Solution
|
- Atomic number is considered to be a more appropriate parameter than at...
Text Solution
|
- Two elements with symbol X (atomic no. 16) and Y (atomic no. 12) are p...
Text Solution
|
- Taking the example of an element of atomic number 16, explain how the ...
Text Solution
|
- Calcium is an element with atomic number 20. Stating reason answer eac...
Text Solution
|