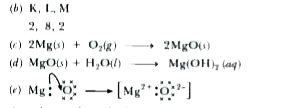Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise HIGHER ORDER THINKING SKILLS|10 VideosPERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (VERY SHORT ANSWER QUESTIONS)|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise SHORT ANSWER QUESTIONS-II|24 VideosMODEL QUESTIONS PAPER - 5
VK GLOBAL PUBLICATION|Exercise SECTION B|3 VideosPRACTICAL BASED QUESTIONS
VK GLOBAL PUBLICATION|Exercise Experiment 15|4 Videos
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -LONG ANSWER QUESTIONS
- How do you calculate the valency of an element from its electronic con...
Text Solution
|
- What is the valency of magnesium with atomic number 12 and sulphur wit...
Text Solution
|
- How does the valency vary in a period on going from left to right?
Text Solution
|
- How does the valency vary in going down a group?
Text Solution
|
- An element is placed in 2^("nd") group and 3^("rd") period of the peri...
Text Solution
|
- Atomic number of few elements are given below 10, 20, 7, 14 (a) Iden...
Text Solution
|
- Mendeleev predicted the existence of certain elements not known at tha...
Text Solution
|
- An element X which is a yellow solid at room temperature shows catenat...
Text Solution
|
- An element X of group 15 exists as diatomic molecule and combines with...
Text Solution
|
- Give an account of the process adopted by Mendeleev for the classifica...
Text Solution
|
- The atomic number of an element ‘X' is 19. (a) Write its electron...
Text Solution
|
- (a) How does the atomic radius change as you go (i) from left to righ...
Text Solution
|
- (a) How would the tendency to lose electrons change as you go (i) fro...
Text Solution
|
- The position of eight elements in the Modern Periodic Table is given b...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of seven elements A, B, C, D, E, F and G have a different number...
Text Solution
|
- In the following table, six elements A, B, C, D, E and F of the modern...
Text Solution
|