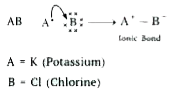Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (VERY SHORT ANSWER QUESTIONS)|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (SHORT ANSWER QUESTIONS-I)|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
VK GLOBAL PUBLICATION|Exercise LONG ANSWER QUESTIONS|17 VideosMODEL QUESTIONS PAPER - 5
VK GLOBAL PUBLICATION|Exercise SECTION B|3 VideosPRACTICAL BASED QUESTIONS
VK GLOBAL PUBLICATION|Exercise Experiment 15|4 Videos
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -HIGHER ORDER THINKING SKILLS
- Using the part of the Periodic Table given below, answer the questions...
Text Solution
|
- Is it possible to have an element with atomic number 2.5?
Text Solution
|
- An element 'X' has mass number 35 and number of neutrons 18. Write ato...
Text Solution
|
- Three elements A, B and C have 3,4 and 2 electrons respectively in the...
Text Solution
|
- Compare the radii of two species X and Y. Give reasons for your answer...
Text Solution
|
- Write the formula of the product formed when the element A (atomic num...
Text Solution
|
- An element X (atomic number 17) reacts with an element Y (atomic numbe...
Text Solution
|
- (a) Electropositive nature of the element(s) increases down the group ...
Text Solution
|
- In the following table, the positions of six elements A, B, C, D, E an...
Text Solution
|
- Two elements ‘A’ and 'B' belong to the 3rd period of Modern periodic t...
Text Solution
|