Similar Questions
Explore conceptually related problems
Recommended Questions
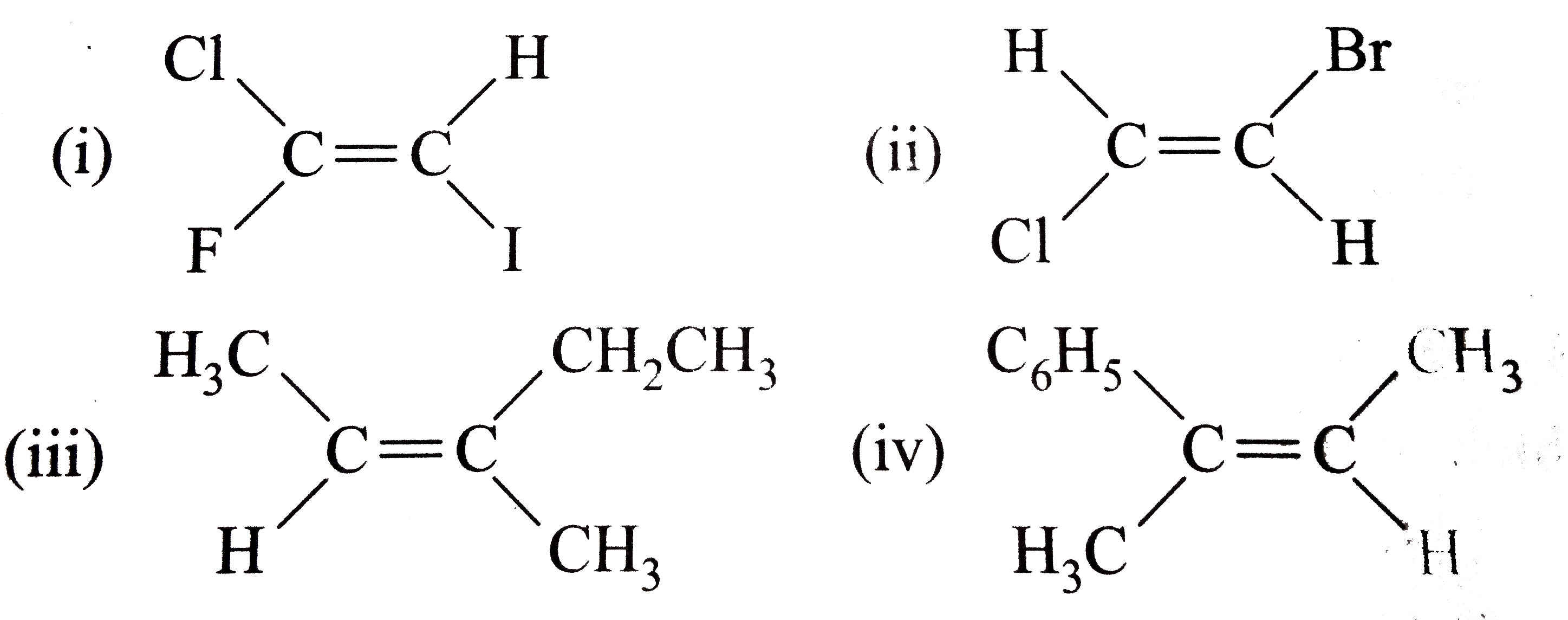
- Classify the following alkenes and their derivatives as Z or E (i) ...
Text Solution
|
- Assertion: Trans isomers are more stable than cis isomers Reason: Th...
Text Solution
|
- Draw geometrical (or cis-and trans-) isomers of the following structur...
Text Solution
|
- Classify the following alkenes and their derivatives as Z or E (i) (ii...
Text Solution
|
- Cis and trans isomers generally
Text Solution
|
- Which of the following statement is not true regarding the cis and tra...
Text Solution
|
- Which of the following alkene on catalytic hydrogenation gives cis and...
Text Solution
|
- How many statements are true for the following pair of compounds ? ...
Text Solution
|
- Draw the cis, trans isomers for the following and designate them as E ...
Text Solution
|