A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
MTG-WBJEE|Exercise WBJEE/WORKOUT (Category 2: Single Option Correct Type)|13 VideosCOORDINATION COMPOUNDS
MTG-WBJEE|Exercise WBJEE/WORKOUT (Category 3: One or More than one Option Correct Type)|7 VideosCHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)|1 VideosENVIRONMENTAL CHEMISTRY
MTG-WBJEE|Exercise WB JEE Previous Years Questions ( CATEGORY 1 : Single Option Correct Type )|2 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-COORDINATION COMPOUNDS-WB JEE Previous Years Questions (CATEGORY 3; ONE OR MORE THAN ONE OPTION CORRECT TYPE(
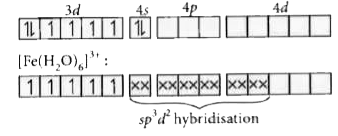
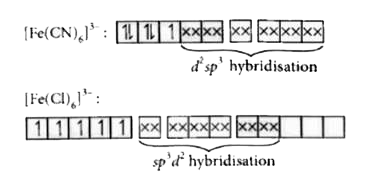
- In [Fe(H(2)O)(6)]^(3+), [Fe(CN)(6)]^(3-), [Fe(Cl)(6)]^(3-) species, th...
Text Solution
|
- In basic medium the amount of Ni^(2+) in a solution can be estimated w...
Text Solution
|
- Optical isomerism is exbibited by (ax= oxalate anion, en=ethylenediami...
Text Solution
|
- Compounds with spin only magnetic moment equivalent to five unpaired ...
Text Solution
|