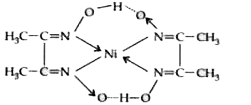
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (Category 1: Single Option Correct Type)|6 VideosCHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)|1 VideosENVIRONMENTAL CHEMISTRY
MTG-WBJEE|Exercise WB JEE Previous Years Questions ( CATEGORY 1 : Single Option Correct Type )|2 Videos
Similar Questions
Explore conceptually related problems