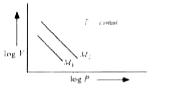
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER (SOLIDS, LIQUIDS AND GASES)
MTG-WBJEE|Exercise WB JEE Previous Years Questions (Category 2: Single Option Correct Type)|3 VideosSTATES OF MATTER (SOLIDS, LIQUIDS AND GASES)
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 3; ONE OR MORE THAN ONE OPTION CORRECT TYPE(|1 VideosSTATES OF MATTER (SOLIDS, LIQUIDS AND GASES)
MTG-WBJEE|Exercise WBJEE/WORKOUT (Category 3: One or More than one Option Correct Type)|9 VideosREDOX EQUILIBRIA
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS|5 VideosSURFACE CHEMISTRY
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS|3 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-STATES OF MATTER (SOLIDS, LIQUIDS AND GASES)-WB JEE Previous Years Questions (Category 1: Single Option Correct Type)
- A vander Waals' gas may behave ideally when
Text Solution
|
- Four gases P,Q,R and S have almost same values of b but their a values...
Text Solution
|
- At a certain temperature the time required for the complete diffusion ...
Text Solution
|
- For one mole of an ideal gas the slope of V vs. T curve at constant pr...
Text Solution
|
- The rms velocity of CO gas molecules at 27^(@)C is approximately 1000 ...
Text Solution
|
- A gas can be liquefied at temperature T and pressure P provided
Text Solution
|
- Suppose the mass of a single Ag atom is m. Ag metal crystallizes in fc...
Text Solution
|
- The units of surface tension and viscosity of liquids are respectively
Text Solution
|
- Ionic solids with Schottky defect may contain in their structure
Text Solution
|
- Which of the following has the dimension of ML^(0)T^(-2)?
Text Solution
|
- For same mass of two different ideal gases of molecular weights M(1) a...
Text Solution
|
- A compound formed by elements X and y crystallises in the cubic struct...
Text Solution
|
- Equal weights of ethane and hydrogen and mixed in an empty container a...
Text Solution
|
- For an van der Waals' gas, the term ((ab)/(V^(2))) represents some
Text Solution
|