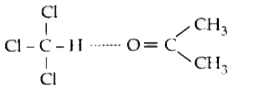
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PHYSICAL CHEMISTRY OF SOLUTIONS
MTG-WBJEE|Exercise WB JEE WORKOUT (One or More than One Option Correct Type )(2 mark)|10 VideosPHYSICAL CHEMISTRY OF SOLUTIONS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (Single Option Correct Type)(1 Mark)|16 VideosPHYSICAL CHEMISTRY OF SOLUTIONS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (Single Option Correct Type)(2 Mark)|2 VideosMODEL TEST PAPTER
MTG-WBJEE|Exercise MCQs|80 VideosPOLYMERS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 1 : Single Option Correct Type )|2 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-PHYSICAL CHEMISTRY OF SOLUTIONS-WB JEE WORKOUT (Single Option Correct Type)(2 mark)
- Give A^(@)(1/3 Al^(3+))=63 Omega^(-1) cm^(2) mol^(-1) and Lambda^(@)(1...
Text Solution
|
- Molar conductivities of Li^(-),Na^(+),K^(+) and Rb^(+) ions in aqueous...
Text Solution
|
- How much amount of KCl must be added to 1 kg of water so that the free...
Text Solution
|
- The freezing point of water is depressed by 0.37^@C in a 0.01 molal Na...
Text Solution
|
- An electrolyte
Text Solution
|
- The cell constant for an electrical conductivity cell having two elect...
Text Solution
|
- The volume of a colloidal particle, VC cas compared to the volume of a...
Text Solution
|
- Which of the following will show a negative deviation from Raoult's la...
Text Solution
|
- A current of 9.65 A is passed for 3 hours between nickel electrodes in...
Text Solution
|
- Lowering of vapour pressure in 1 motal aqueous solution at 100^@C is
Text Solution
|
- A solution prepared by dissolving 15 g of non-volatile solute in 270 g...
Text Solution
|
- A current of 12 ampere is passed through an electrolytic cell containi...
Text Solution
|
- Kohlrausch law states that at
Text Solution
|
- Which of the following will have the highest coagulating power for As(...
Text Solution
|
- On passing a current of 1.0 ampere for 16 min and 5 seconds through 1 ...
Text Solution
|