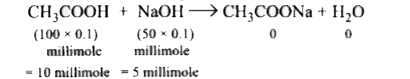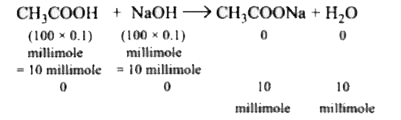A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG-WBJEE-IONIC EQUILIBRIA-WB JEE PREVIOUS YEARS QUESTIONS
- At 25^@ C, the solubility product of a salt of MX2 type is 3.2 xx 10^...
Text Solution
|
- The different colours of litmus in acidic, neutral and basic solutions...
Text Solution
|
- At 25^@ C, pH of a 10^(-8) M aqueous KOH solution will be
Text Solution
|
- The pH of 10^(-4) M KOH solution will be
Text Solution
|
- The ratio of volumes of CH3 COOH 0.1 (N) to CH3 COONa 0.1 (N) required...
Text Solution
|
- The molar solubility (in mol L^(-1)) of a sparingly soluble salt MX4 i...
Text Solution
|
- Dissolving NaCN in de-ionized water will result in a solution having
Text Solution
|
- Which of the following mixtures will have the lowest pH at 298 K?
Text Solution
|
- 1 xx 10^(-3) mole of HCI is added to a buffer solution made up of 0....
Text Solution
|
- In which of the following mixed aqueous solutions pH = pka at equilibr...
Text Solution
|