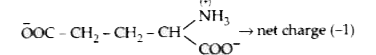
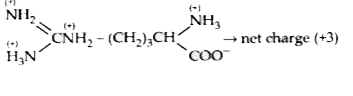
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INTRODUCTION TO BIOMOLECULES
MTG-WBJEE|Exercise WB JEE WORKOUT (ONE OR MORE THAN ONE OPTION CORRECT TYPE)|10 VideosINTRODUCTION TO BIOMOLECULES
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (SINGLE OPTION CORRECT TYPE)|7 VideosHYDROGEN
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS|2 VideosIONIC EQUILIBRIA
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-INTRODUCTION TO BIOMOLECULES-WB JEE PREVIOUS YEARS QUESTIONS (SINGLE OPTION CORRECT TYPE)
- An electric current is passed through an aqueous solution of a mixture...
Text Solution
|
- The optically active molecule is .
Text Solution
|
- The correct structure of the dipeptide gly - ala is .
Text Solution
|
- Ribose and 2 - deoxyribose can be differentiated by .
Text Solution
|
- In DNA, the consecutive deoxynucleotide are connected via.
Text Solution
|
- The number of amino acids and number of peptide bonds in a linear te...
Text Solution
|
- ADP and ATP differ in the number of
Text Solution
|
- Within the list show below , the correct pair of structure of alanin...
Text Solution
|