Similar Questions
Explore conceptually related problems
Recommended Questions
- The complexes given below are –
Text Solution
|
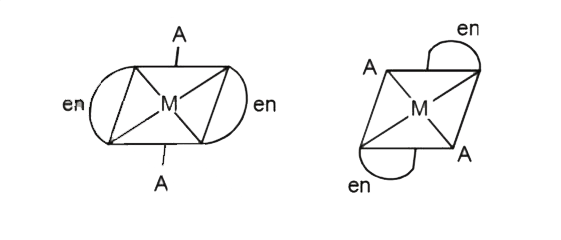
- The Complexes given below show:
Text Solution
|
- The two complexes given given below are
Text Solution
|
- The two complexes given below are
Text Solution
|
- The complexes given below show:
Text Solution
|
- Complexes given below show:
Text Solution
|
- Select the correct statement regarding the complex given below :
Text Solution
|
- The complexes given below show : " and "
Text Solution
|
- The complexes given below are –
Text Solution
|