Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
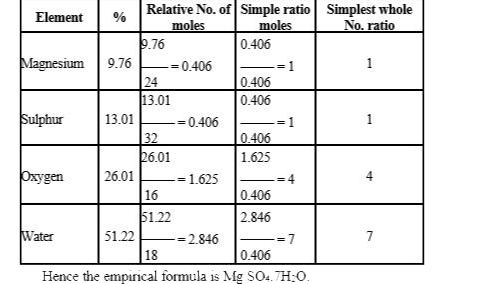
- A compound has the following composition Mg = 9.76%,S = 13.01%, 0 = 26...
Text Solution
|
- एक यौगिक की प्रतिशत रचना निम्नलिखित है- हाइड्रोजन =2.48% गन्धक =39....
Text Solution
|
- An oxide of nitrogen has the percentage composition : Fe = 36.76 , S =...
Text Solution
|
- A sample of a salt has the percentage composition : Fe = 36.76 , S = 2...
Text Solution
|
- A salt containing water of crystallization gave the following percenta...
Text Solution
|
- A compound has the following composition Mg = 9.76%, S = 13.01 %, O=26...
Text Solution
|
- एक यौगिक का प्रतिशत संघटन निम्न प्रकार है, इसका मूलानुपाती सूत्र ज्ञात...
Text Solution
|
- In MgSO(4) (at. mass : Mg = 24, S = 32, O = 16), the mass percentage o...
Text Solution
|
- In MgSO(4) (At. Mass: Mg = 24, S = 32, O = 16), the mass percentage of
Text Solution
|