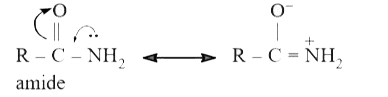
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain why amines are more basic than amides.
Text Solution
|
- मेथिल ऐमीन अमोनिया से अधिक क्षारकीय है, समझाइए।
Text Solution
|
- एथिल ऐमीन, ऐनिलीन से अधिक क्षारकीय है, समझाइए।
Text Solution
|
- कथन : ऐमाइड्स ऐमीन्स की अपेक्षा अधिक क्षारकीय होते हैं। कारण : ऐमाइड...
Text Solution
|
- Why is an amide more acidic than amine?
Text Solution
|
- Amines are more basic than
Text Solution
|
- Explain why amines are more basic than amides.
Text Solution
|
- Account for the following Amines are more basic than amides.
Text Solution
|
- Why is an amide more acidic than amine?
Text Solution
|