Similar Questions
Explore conceptually related problems
Recommended Questions
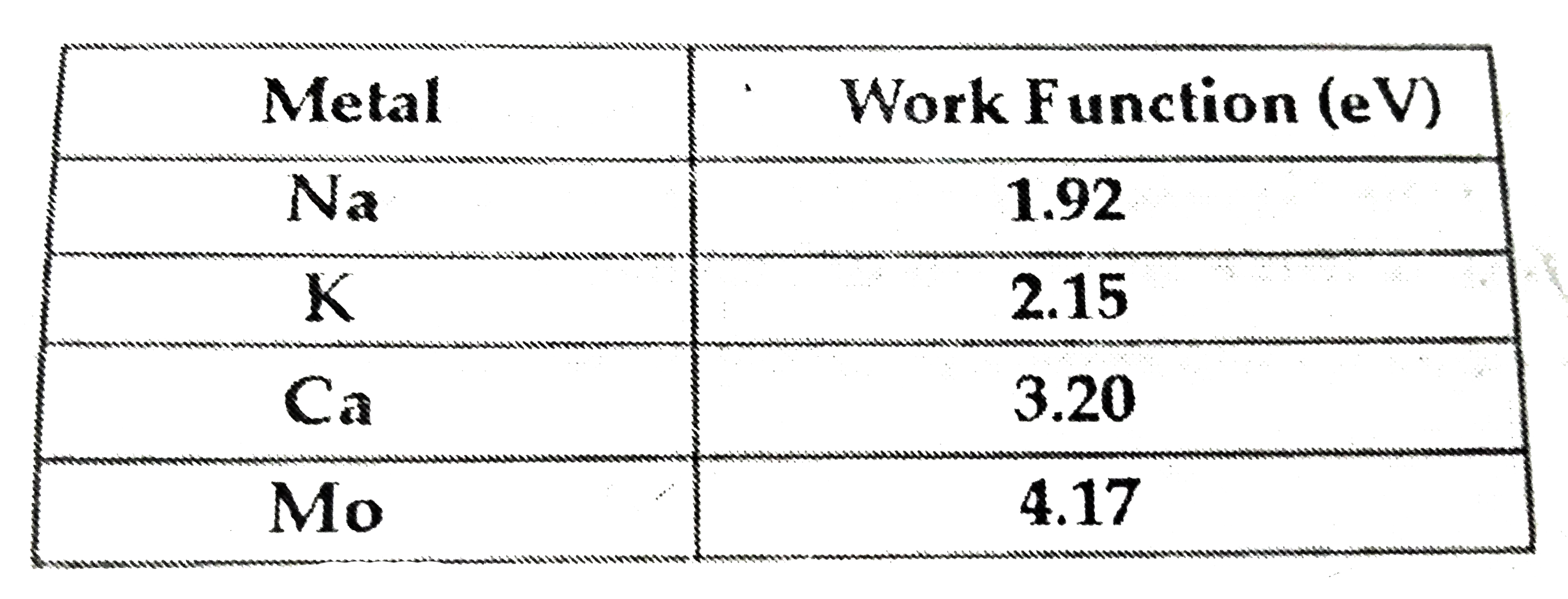
- If light of wavelength 412.5 nm is incident on each of the metals give...
Text Solution
|
- The work function (phi) of some metals is listed below . The number of...
Text Solution
|
- If light of wavelength 412.5 nm is incident on each of the metals give...
Text Solution
|
- The work function for a certain metal is 4.2 eV. Will this metal give ...
Text Solution
|
- If light of wavelength 412-5 nm is indicent on each of the metals give...
Text Solution
|
- The work function for a certain metal is 4.2 eV. Will this metal give ...
Text Solution
|
- The work function for a certain metal is 4.2 eV. Will this metal give ...
Text Solution
|
- The work function for a certain metal is 4.2 eV. Will this metal give ...
Text Solution
|
- The work function for a certain metal is 4.2 eV. Will this metal give ...
Text Solution
|