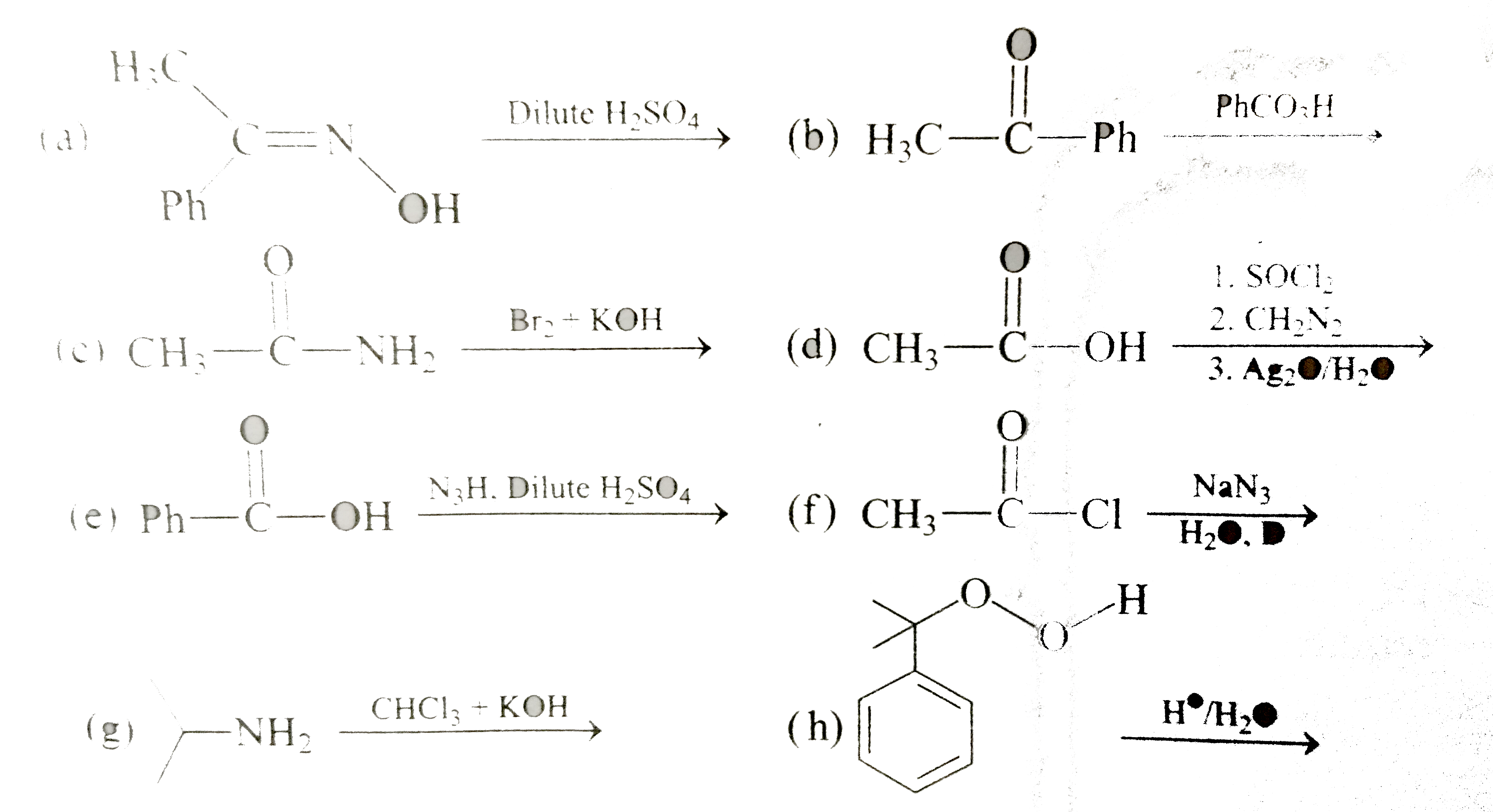
Similar Questions
Explore conceptually related problems
Recommended Questions
- Find out number of reaction which involve electron deficient nitrogen ...
Text Solution
|
- Reactions Involving Displacement Of Nitrogen
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Find out number of reaction which involve electron deficient nitrogen ...
Text Solution
|
- Which of the following mechanism is/are not seen during esterification...
Text Solution
|
- The reaction that is NOT involved in the ozone layer depletion mechani...
Text Solution
|
- During conversion of oxime to nitroparaffins, which reaction is involv...
Text Solution
|
- अभिक्रिया में सम्मिलित इलेक्ट्रॉनस्नेही है
Text Solution
|
- Explain the mechanism involved in the reaction of phosphorous trichlor...
Text Solution
|