A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
MTG-WBJEE|Exercise WB JEE WORKOUT ( CATEGORY 3 : One or Mare than One Option Correct Type (2 Mark )|10 VideosTHERMODYNAMICS
MTG-WBJEE|Exercise WB JEE Previous Years Questions|12 VideosTHERMODYNAMICS
MTG-WBJEE|Exercise WB JEE Previous Years Questions|12 VideosSOLID STATE ELECTRONS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 1 : Single Option Correct Type)|15 VideosWAVE OPTICS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTION (MCQ.s)|9 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-THERMODYNAMICS-WB JEE WORKOUT ( CATEGORY 2 : Single Option Correct Type (2 Mark )
- Three moles of an ideal monoatomic gas perform a cycle shown in figure...
Text Solution
|
- Figure shows the variation of internal energy (U) with the pressure (P...
Text Solution
|
- A diatomic ideal gas is heated at constant at constant volume until th...
Text Solution
|
- Oxygen gas is made to undergo a process in which its molar heat capaci...
Text Solution
|
- A gass of given mass at a pressure of 10^(5)Nm^(-2) expands isothermal...
Text Solution
|
- A Carnot engine whose sink is at 300 K has an efficiency of 40 %. By h...
Text Solution
|
- A gas expands with temperature according to the relation V=KT^(2/3).Wo...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic process abca, as shown in ...
Text Solution
|
- A diatomic ideal gas undergoes a thermodynainic change according to th...
Text Solution
|
- An ideal gas is taken from state A to state B following three differen...
Text Solution
|
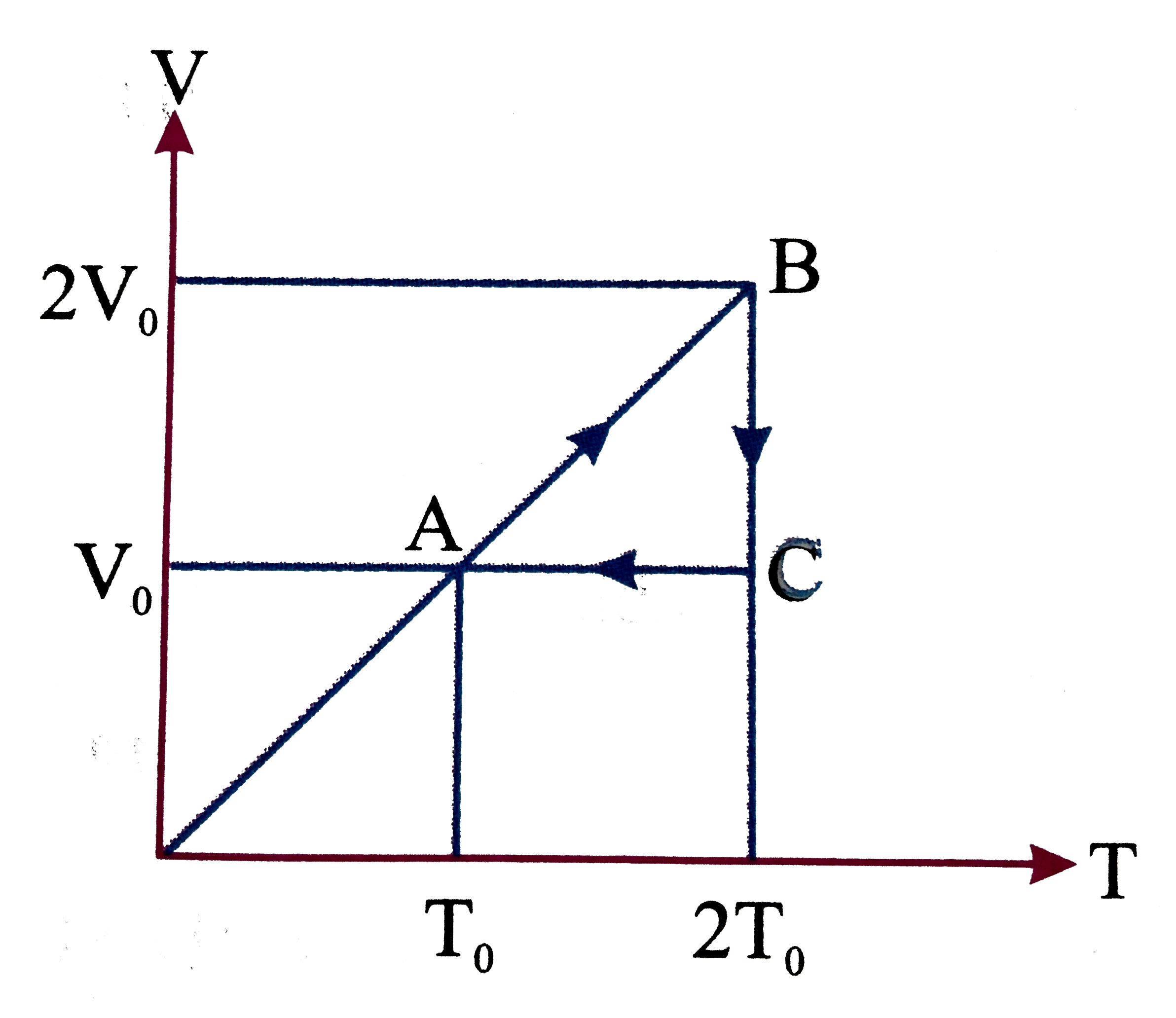
- A cyclic process ABCA is shown in the given V-T diagram. Process in th...
Text Solution
|
- A petrol engine consumes 20 kg of petrol per hour. The calorific value...
Text Solution
|
- A thermodynamic process of one mole ideal monatomic gas 2.is shown in...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- The temperature -entropy diagram of a reversible engine cycle is given...
Text Solution
|