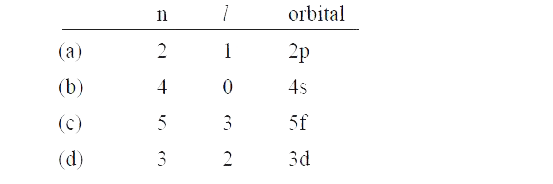Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE-I
NCERT TAMIL|Exercise QUESTIONS (A . CHOOSE THE BEST ANSWER )|13 VideosATOMIC STRUCTURE-I
NCERT TAMIL|Exercise QUESTIONS (B.FILL UP THE BLANKS)|5 VideosAROMATIC HYDROCARBONS
NCERT TAMIL|Exercise QUESTION|24 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
NCERT TAMIL|Exercise EVALUATION (Write brief answer to the following questions) |19 Videos
Similar Questions
Explore conceptually related problems
NCERT TAMIL-ATOMIC STRUCTURE-I-QUESTIONS (D. EXPLAIN BRIEFLY ON THE FOLLOWING)
- Using s, p, d, f notations, describe the orbital with the following qu...
Text Solution
|
- Describe Aufbau principle. Explain its significance in the electronic ...
Text Solution
|
- Using the s, p, d, notation, describe the orbital with the following q...
Text Solution
|
- Using the a Aufbau principle, write the electronic configuration in th...
Text Solution
|
- What is Rutherford’s alpha- ray scattering experiment? What are its co...
Text Solution
|
- What are the postulates of Bohr theory of atom?
Text Solution
|
- Explain the various quantum numbers which completely specify the elect...
Text Solution
|