A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE- PRACTICE SHEET (ADVANCED) (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|2 VideosSTATES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE-PRACTICE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE- I)|3 VideosSTATES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL-II LECTURE SHEET (ADVANCED)) (INTEGER TYPE QUESTIONS)|2 VideosSOLUTIONS
AAKASH SERIES|Exercise Objective Exercise -4|50 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) (INTEGER TYPE QUESTION)|2 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STATES OF MATTER -ADDITIONAL PRACTICE EXERCISE- PRACTICE SHEET (ADVANCED) (STRAIGHT OBJECTIVE TYPE QUESTIONS)
- 2gm of 'He' exerts a pressure of 2 atmospheres in a rigid flask. How m...
Text Solution
|
- If the above tube is kept in vertical position by how much length the ...
Text Solution
|
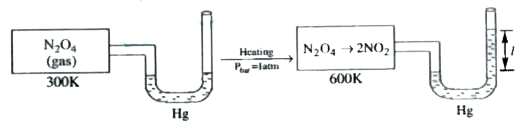
- If the value of 7' in the above diagram is 114 cm. What is the percent...
Text Solution
|
- The compression factor (compressibility factor) for one mole of a van ...
Text Solution
|
- Using van der Waals equation, calculate the constant, a when two moles...
Text Solution
|
- A graph is plotted between PV(m) along y-axis and P along x-axis, whe...
Text Solution
|
- At 400 K, the root mean square (rms) speed of a gas X (molecular weigh...
Text Solution
|
- One way of writing the equation of state for real gases is PV = RT [1+...
Text Solution
|
- Calculate the pressure exerted by one moe of CO(2) gas at 273 K if th...
Text Solution
|
- If 250 ml. of N2 over water at 30°C and a total pressure of 740 torr i...
Text Solution
|