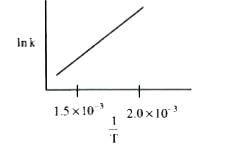
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE -II) (LEVEL - II) STRAIGHT OBJECTIVE TYPE QUESTIONS|20 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE -II) (LEVEL - II) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS|10 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE -I) LEVEL-II (MATRIX MATCHING TYPE QUESTIONS )|5 VideosCARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise CONVERSIONS|19 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL EQUILIBRIUM-PRACTICE SHEET (EXERCISE -II) (LEVEL - I) STRAIGHT OBJECTIVE TYPE QUESTIONS
- A((s)) + B((g)) + "heat" hArr 2C((s)) + 2D((g)) . At equilibrium the p...
Text Solution
|
- For the reaction PCl(5(g)) hArr PCl(3(g)) + Cl(2(g)) the forward reac...
Text Solution
|
- In the reaction N(2(g)) + O(2(g)) hArr 2NO((g)) , Delta H = + 180 kJ...
Text Solution
|
- For the Chemical reaction A(2(g)) + B(2(g)) hArr 2 AB(g) the amount o...
Text Solution
|
- High temperature and high pressure (as per Lechatclier principle) favo...
Text Solution
|
- For CaCO(3)(S) hArr CaO(s) + CO(2) (g), Delta H = + Q at equilibrium. ...
Text Solution
|
- Yield of Ammonia will be more in Haber's process under conditions (L ...
Text Solution
|
- K(C) "for " H(2) + 1//2 O(2) rArr H(2) O at 500 K is 2.4 xx 10^(47) . ...
Text Solution
|
- With increase in temperature generally the value of the equilibrium co...
Text Solution
|
- Le chatelier's principle is applicable to
Text Solution
|
- In the dissociation of CaCO(3) in a closed vessel, the forward reactio...
Text Solution
|
- K(C) value of a gaseous reaction is 5mole/lit. If pressure is increase...
Text Solution
|
- K(p) = 1, For the equilibrium CaCO(3(s)) hArr CaO((s)) + CO(2(g)). The...
Text Solution
|
- The graph relates in K(eq)."Vs" (l)/(T) for a reaction. The reaction m...
Text Solution
|
- Choose the incorrect statement .
Text Solution
|
- For the equilibrium, N(2) O(4) hArr 2NO(2) , (G^(@)N(2)O(4))(298) = 10...
Text Solution
|
- At 627^(@) C and one atmosphere SO(3) is is partially dissociated int...
Text Solution
|