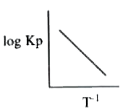
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II) (LECTURE SHEET (ADVANCED))INTEGER TYPE QUESTIONS|5 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED)(STRAIGHT OBJECTIVE TYPE QUESTIONS )|20 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II) (LECTURE SHEET (ADVANCED))STRAIGHT OBJECTIVE TYPE QUESTIONS|20 VideosCARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise CONVERSIONS|19 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL EQUILIBRIUM-ADDITIONAL PRACTICE EXERCISE (LEVEL -II) (LECTURE SHEET (ADVANCED))MATRIX MATCHING TYPE QUESTIONS
- When two reactants A and B are mixed to give products C and D, the rea...
Text Solution
|
- Plots of log K Vs (1)/(T) plots shows an intercept of 2 on y-axis with...
Text Solution
|
- Lechatelier's principle is
Text Solution
|
- N(2) O(2) hArr 2NO, K(1) , (1)/(2) N(2) + (1)/(2) O(2) hArr NO. K(2) ,...
Text Solution
|
- In what manner will increase of pressure affect the following equation...
Text Solution
|
- Variation of K(p) with temperature T is given by for the following cqu...
Text Solution
|
- Select the correct relationship for the given eqilibrium
Text Solution
|
- For the reaction, N(2) + 3H(2) hArr 2NH(3) in a vessel, equal moles o...
Text Solution
|
- In the dissociation of 2Hl hArr H(2) +l(2), the degree of dissociaito...
Text Solution
|
- For the cqiuilibrium LiCI 3NH(3(s)) LiCI NH(3(s)) + 2NH(3(g)), Kp = 9...
Text Solution
|