Similar Questions
Explore conceptually related problems
Recommended Questions
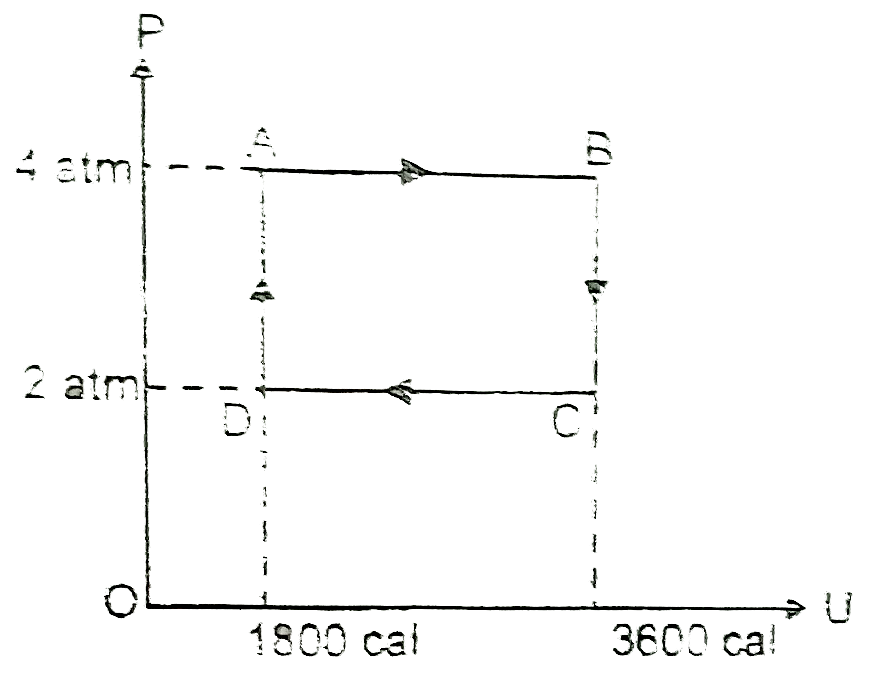
- Two moles of an ideal monoatomic gas undergo a cyclic process which is...
Text Solution
|
- An ideal monoatomic gas undergoes a process in which its internal ener...
Text Solution
|
- One mole of an ideal gas has an interal energy given by U=U(0)+2PV , w...
Text Solution
|
- Two moles of an ideal monoatomic gas undergo a cyclic process which is...
Text Solution
|
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- An ideal gas undergoes cyclic process ABCDA as shown in givend p-V dia...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA a...
Text Solution
|
- An ideal gas undergoes a thermodynamic process in which internal energ...
Text Solution
|
- An ideal gas undergoes a thermodynamic process in which internal energ...
Text Solution
|