Similar Questions
Explore conceptually related problems
Recommended Questions
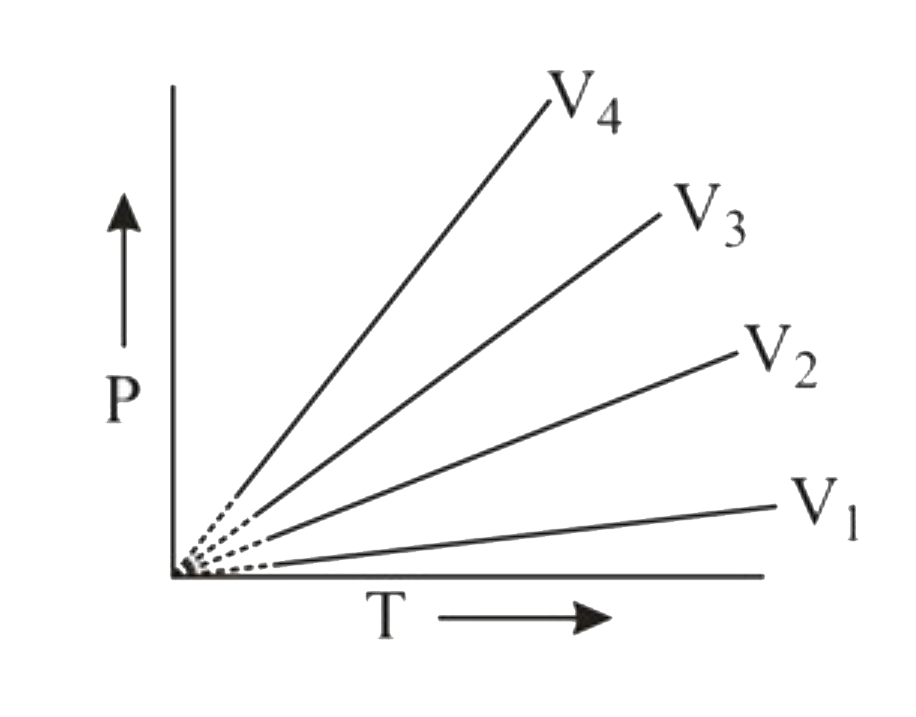
- The ideal gas equation for 1 mol of ideal gas is PV = nRT graph betw...
Text Solution
|
- For the given ideal gas equation PV=nRT, answer the following question...
Text Solution
|
- For the given ideal gas equation PV=nRT , answer the following questio...
Text Solution
|
- For the given ideal gas equation PV=nRT , answer the following questio...
Text Solution
|
- A graph is plotted between p (atm) vs t^(@)C for 10 mol of an ideal ga...
Text Solution
|
- A plot of P vs T for a given mass of gas at constant volume is a strai...
Text Solution
|
- The ideal gas equation for 1 mol of ideal gas is PV = nRT graph betw...
Text Solution
|
- Establish ideal gas equations (PV=nRT).
Text Solution
|
- For an ideal gas the graph between PV/RT and T is
Text Solution
|