Similar Questions
Explore conceptually related problems
Recommended Questions
- For H-atoms , the energy required for the removal of electron from var...
Text Solution
|
- The energy required in ionise a helium atom is equal to 24.6 eV The ...
Text Solution
|
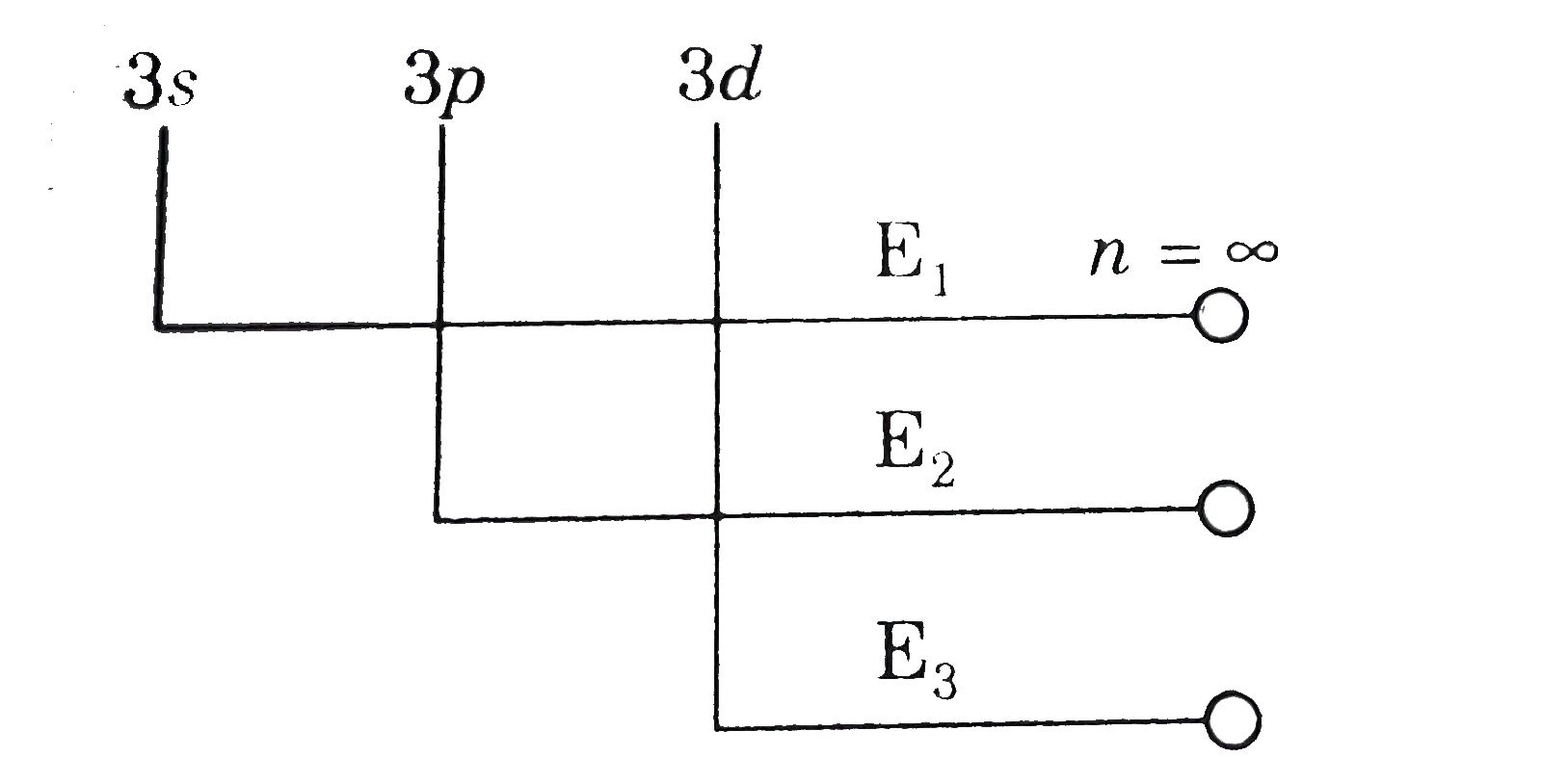
- In hydrogen atom, the order of energies of sub-shell of third energy...
Text Solution
|
- One requires energy En to remove a nucleon from a nucleus and an energ...
Text Solution
|
- How much energy is required to ionise a H - atom if the electron occup...
Text Solution
|
- For H-atoms , the energy required for the removal of electron from var...
Text Solution
|
- The energy required to remove the electron from a singly ionized Hel...
Text Solution
|
- H-परमाणु से एक इलेक्ट्रॉन को निकालने के लिए कितनी ऊर्जा की आवश्यकता हो...
Text Solution
|
- बहु -इलेक्ट्रॉनिक परमाणु // आयनों के किसी कोश में उपस्थित उपकोशों की ऊ...
Text Solution
|