Similar Questions
Explore conceptually related problems
Recommended Questions
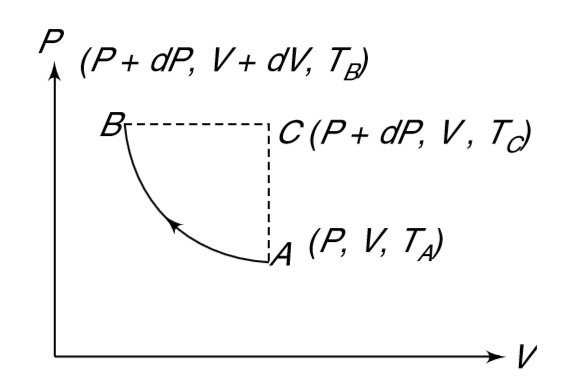
- One mole of a gas is in state A[P, V, T(A)]. A small adiabatic process...
Text Solution
|
- An ideal gas (2.0 moles) is carried round a cycle as shown. If the pro...
Text Solution
|
- P-V curve of a diatomic gas is shown in the Fig. Find the total heat g...
Text Solution
|
- One mole of a monatomic gas is taken from a point A to another point B...
Text Solution
|
- A fixed mass of gas is taken through a process A rarr B rarr C rarrA. ...
Text Solution
|
- A fixed mass of gas is taken through a process A rarr B rarr C rarrA ....
Text Solution
|
- An ideal gas undergoes a series of processes represented bya rarr B ra...
Text Solution
|
- One mole of a gas is in state A[P, V, T(A)] . A small adiabatic proces...
Text Solution
|
- Five moles of gas is put through a series of changes as shown graphica...
Text Solution
|