Similar Questions
Explore conceptually related problems
Recommended Questions
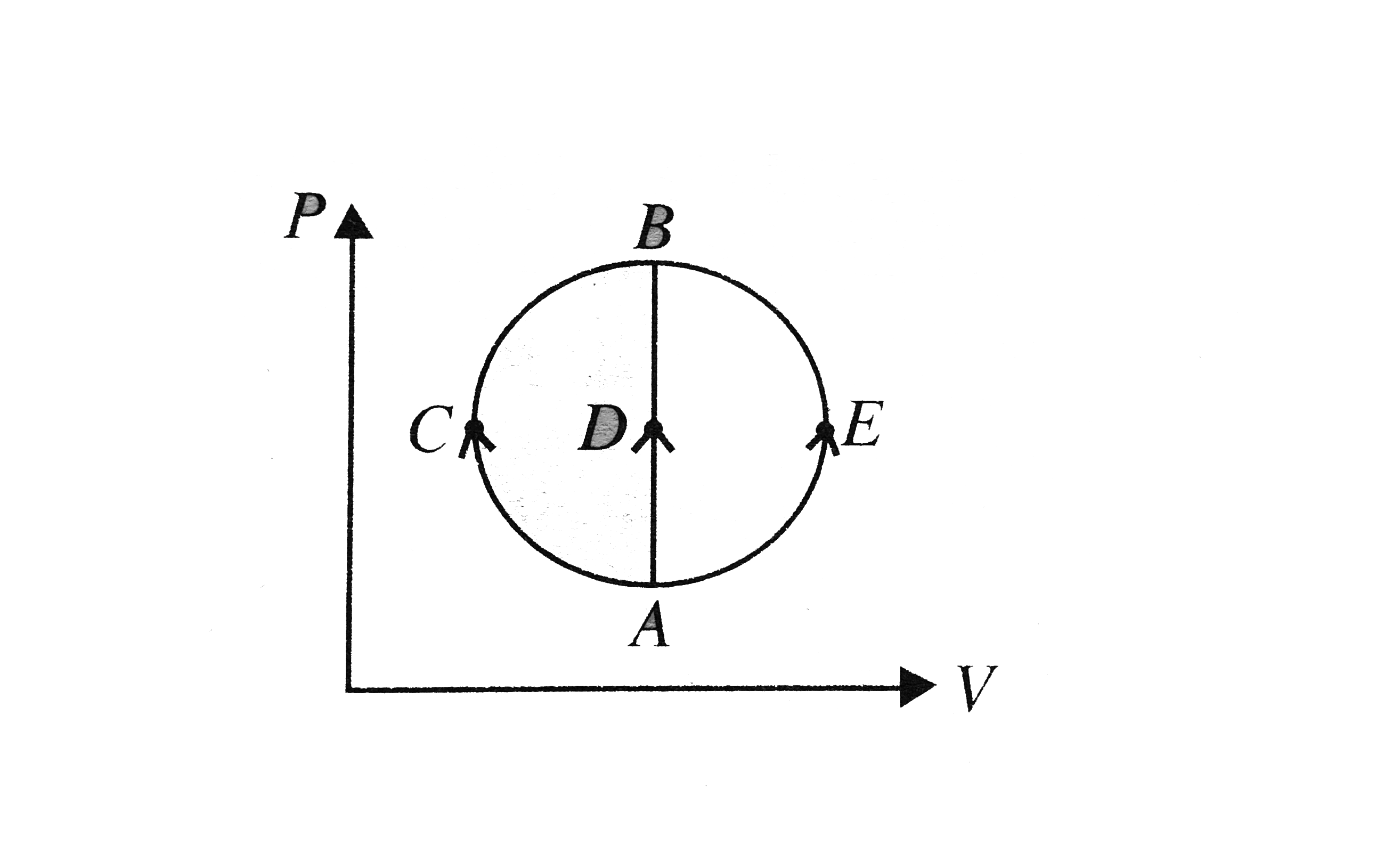
- One mole of an ideal gas is taken from state A to state B by three dif...
Text Solution
|
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- One mole of an ideal gas is taken from state A to state B by three dif...
Text Solution
|
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- An ideal gas taken from state A(P,V) to the state B(P//2,2V) along a s...
Text Solution
|
- An ideal gas is taken from its initial state a to its find state d in ...
Text Solution
|
- One mole of an ideal monoatomic gas is taken through the thermodynamic...
Text Solution
|
- One mole of ideal monoatomic gas is taken round the cyclic process as ...
Text Solution
|
- One mole of monatomic gas is brought from state A to state B. The init...
Text Solution
|