Similar Questions
Explore conceptually related problems
Recommended Questions
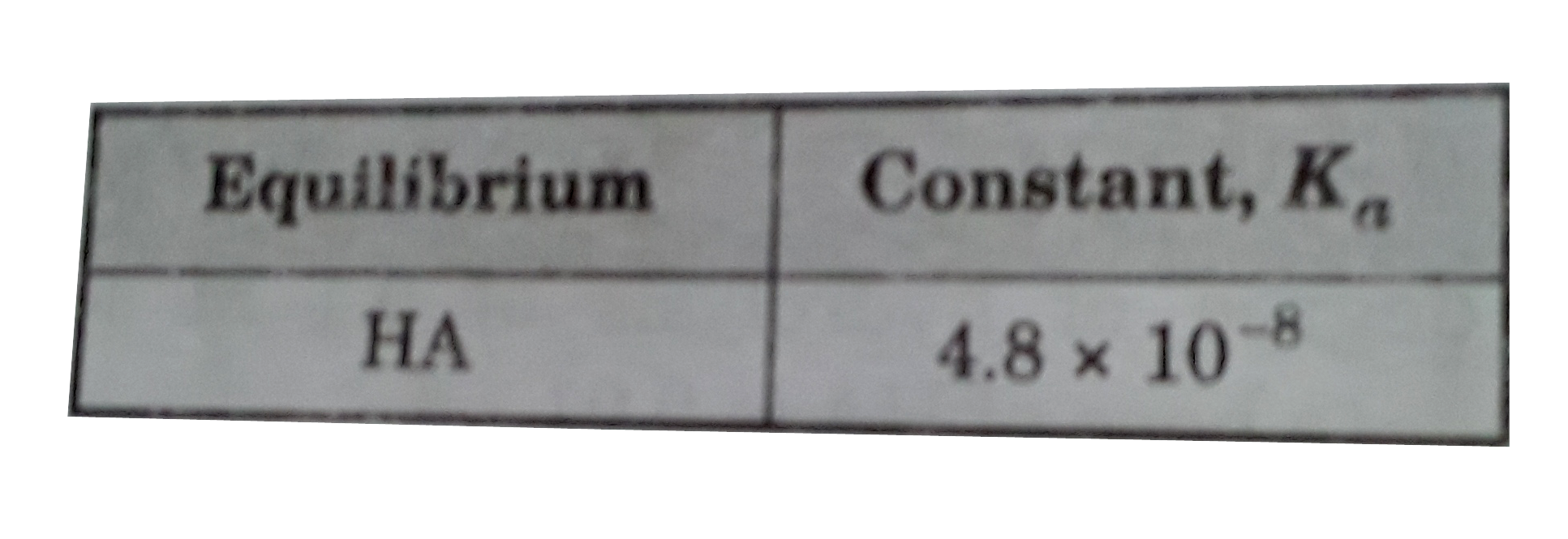
- What is the [H^+] of a 0.075 M solution of the acid HA ?
Text Solution
|
- 0.03 M aqueous solution of a weak monobasic acid solution (K(a)=10^(-1...
Text Solution
|
- A 12.0 M acid solution that contains 75.0% acid by mass has a density ...
Text Solution
|
- A 0.015 M solution of a weak acid has a pH of 3.52 . What is the value...
Text Solution
|
- The ionization of benzoic acid is represented by this equation C6H5COO...
Text Solution
|
- What is the [H^+] of a 0.075 M solution of the acid HA ?
Text Solution
|
- The P^(H) of 0.01 M solution of acetic acid is 5.0 What are the values...
Text Solution
|
- HA is a weak acid. The pH of 0.1 M HA solution is 2. What is the degre...
Text Solution
|
- HA is a weak acid. The pH of 0.1 M HA solution is 2. What is the degre...
Text Solution
|