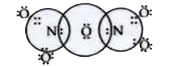
Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise NCERT TEXTBOOK EXERCISES|42 VideosTHE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise CASE BASED/ SOURCE-BASED INTEGRATED QUESTIONS|19 VideosTHE D-AND F-BLOCK ELEMENTS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION A ) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)|7 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-THE P-BLOCK ELEMENTS -SELF ASSESSMENT TEST ( SECTION A)
- What is the covalence of nitrogen in N2O5 ?
Text Solution
|
- Which of the following is not a p-block element ?
Text Solution
|
- Choose the correct option:
Text Solution
|
- Oxygen shows anomalous behaviour because
Text Solution
|
- Hydrogen peroxide on heating gives
Text Solution
|
- Which of the following is not a neutral oxide ?
Text Solution
|
- Assertion (A) : Rhombic sulphur is obtained by evaporating a solution ...
Text Solution
|
- Assertion (A): Noble gases show no tendency for chemical reaction. ...
Text Solution
|