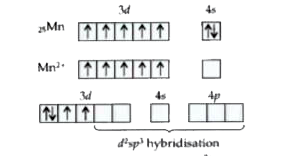
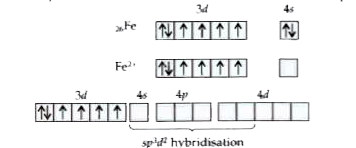
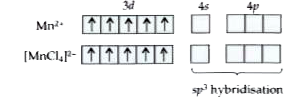
Text Solution
Verified by Experts
Topper's Solved these Questions
THE D-AND F-BLOCK ELEMENTS
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS|15 VideosTHE D-AND F-BLOCK ELEMENTS
U-LIKE SERIES|Exercise MULTIPLE CHOICE QUESTIONS|20 VideosTHE D-AND F-BLOCK ELEMENTS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION A ) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)|7 VideosSURFACE CHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST ( SECTION A)|7 VideosTHE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST ( SECTION A)|7 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-THE D-AND F-BLOCK ELEMENTS -NCERT TEXTBOOK EXERCISES
- For M^(2+)//M and M^(3+)//M^(2+) systems, the E^(@) values for some me...
Text Solution
|
- Predict which of the following will be coloured in aqueous solution ? ...
Text Solution
|
- Compare the stability of +2 oxidation state for the elements of the fi...
Text Solution
|
- Compare the chemistry of actinoids with that of the langthanoids with ...
Text Solution
|
- How would you account for the following : (i) Of the d^(4) species, ...
Text Solution
|
- What is meant by 'disproportionation'? Give two examples of disproport...
Text Solution
|
- Which metal in the first series of transition metals exhibits +1 oxida...
Text Solution
|
- Calculate the number of unpaired electrons in the following gaseous io...
Text Solution
|
- Give examples and suggest reasons for the following features of the tr...
Text Solution
|
- What are alloys ? Name an important alloy which contains some of the l...
Text Solution
|
- What are inner transition elements ? Decide which of the following ato...
Text Solution
|
- The chemistry of the actinoid elements is not so smooth as that of the...
Text Solution
|
- Which is the last element in the series of the actinoids ? Write the e...
Text Solution
|
- Use Hund's rule to derive the electronic configuration of Ca^(3+) ion,...
Text Solution
|
- Name the members of the lanthanoid series which exhibit +2 oxidation s...
Text Solution
|
- Write the electronic configurations of the elements with atomic number...
Text Solution
|
- Compare the general characteristics of the first series of transition ...
Text Solution
|
- Write down the number of 3d electrons in each of the following ions : ...
Text Solution
|
- Comment on the statement that elements of the first transition series ...
Text Solution
|
- What can be inferred from the magnetic moment values of the following ...
Text Solution
|