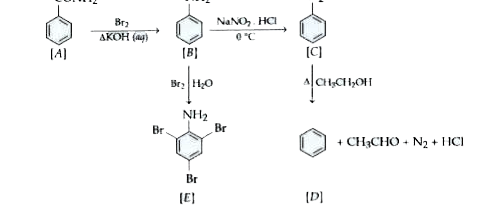
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-SAMPLE QUESTIONS PAPER -SECTION D
- The e.m.f of the follwing cell at 198k is 0.1745V, Fe(s)//Fe^(2+)(0....
Text Solution
|
- (a) Calculate the degree of dissociation 'A' and 'B' are dilute . The ...
Text Solution
|
- An organic compound 'A' with molecular formula C(7)H(7)NOreacts with B...
Text Solution
|
- (a) How will you convert: (i) Aniline into fluorobenzene. (ii) Ben...
Text Solution
|
- (a) when a chromite ore (A) is fused with an aqueous solution of sodiu...
Text Solution
|
- what happens when : (i) Manganate ions (MnO(4)^(2)) undergoes dispro...
Text Solution
|