Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATISTICS
NCERT BANGLISH|Exercise Exercise 14.2|6 VideosSTATISTICS
NCERT BANGLISH|Exercise Exercise 14.3|7 VideosSTATISTICS
NCERT BANGLISH|Exercise THINK AND DISCUSS|8 VideosSIMILAR TRIANGLES
NCERT BANGLISH|Exercise TRY THIS|6 VideosTANGENTS AND SECANTS TO A CIRCLE
NCERT BANGLISH|Exercise Try this|5 Videos
Similar Questions
Explore conceptually related problems
NCERT BANGLISH-STATISTICS-Exercise 14.1
- A survey conducted by a group of students as a part of their environme...
Text Solution
|
- Consider the following distribution of daily wages of 50 workers of a ...
Text Solution
|
- The following distribution shows the daily pocket allowance of childre...
Text Solution
|
- Find True or False: 15 is the mode of the data 16,15,17,16,15,19,17,14...
Text Solution
|
- In a retail market, fruit vendors were selling oranges kept in packing...
Text Solution
|
- The table below the daily expenditure on food of 25 household in a loc...
Text Solution
|
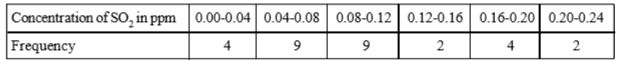
- To find out the concentration of SO(2) in the air (in parts per millio...
Text Solution
|
- Find True or False: 18 is the mode of the data 3,14,18,21,14,18.
Text Solution
|
- The following table gives the literacy rate (in percentage) of 35 citi...
Text Solution
|