Similar Questions
Explore conceptually related problems
Recommended Questions
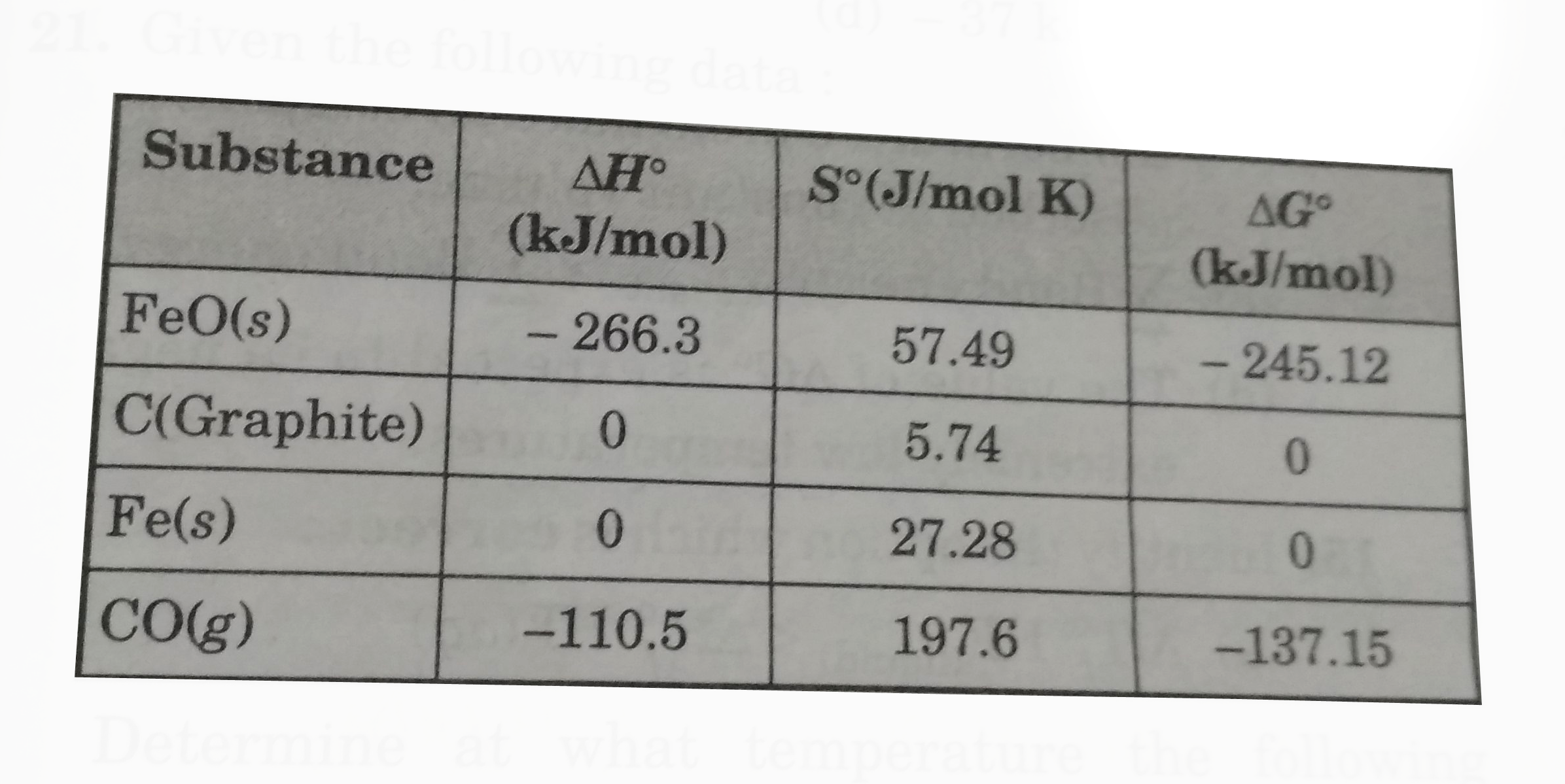
- Given the following data : Determine at what temperature the foll...
Text Solution
|
- From the data at 25^(@)C : Fe(2)O(3)(s) +3C(("graphite")) rarr 2Fe(s) ...
Text Solution
|
- Given the following data : Determine at what temperature the following...
Text Solution
|
- From the following data at 25^(@)C, Calculate the standard enthalpy of...
Text Solution
|
- Given the following data {:("Substance",DeltaH^(@)(KJ//mol),S^(@)(J/...
Text Solution
|
- Given the following data: {:("Substance", DeltaH^(@)"(KJ//mol)", S^(@)...
Text Solution
|
- निम्न दो अभिक्रियाओं ज्ञात है FeO(s)+3CO(g)to2Fe(s)+3CO(2)(g)," "...
Text Solution
|
- Determine the standard enthalpy of formation of FeO(s) and Fe(2)(O(3)(...
Text Solution
|
- In the case of the following reactions Kp Oh Kc Determine the relation...
Text Solution
|