Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT GUJARATI-CHEMICAL KINETICS - I-Questions(E. Explain briefly on the following)
- Discuss the rate of the reaction 2N(2)O(5(g)) rarr 4NO(2(g))+O(2(g))
Text Solution
|
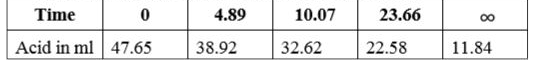
- One ml of methyl acetate was added to 20 ml of 0.5 N sulphuric acid. 2...
Text Solution
|
- In I order reaction the initial concentration of the reactant as 0.05 ...
Text Solution
|
- If a reaction with t1//2=69.3 second, has a rate constant value of 10^...
Text Solution
|
- The time for half life of a first order reaction is 1 hr. what is the ...
Text Solution
|
- The following results were obtained for the saponification of ethyl ac...
Text Solution
|