Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT GUJARATI-KINETIC THEORY-ADDITIONAL EXERCISE
- A metre long narrow bore held horizontally (and closed at one end) con...
Text Solution
|
- From a certain apparatus, the diffusion rate of hydrogen has an averag...
Text Solution
|
- A gas in equilibrium has uniform density and pressure throughout its v...
Text Solution
|
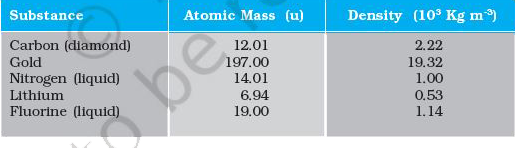
- Given below are densities of some solids and liquids. Give rough estim...
Text Solution
|