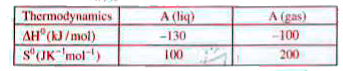A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYNAMICS-Additional Practice Exercise
- underset(P(1), V(1), T(1))("State") A overset("Rev")rarr underset(P(2)...
Text Solution
|
- During winters, moisture condenses in the form of dew and can be seen ...
Text Solution
|
- Assuming Delta H^(0) and Delta S^(0) do not change with temperature th...
Text Solution
|
- For the reaction X(2)O(4(l)) rarr 2XO(2(g)), Delta U= 2.1 "kcal", Delt...
Text Solution
|
- The correct signs of Delta S for the following four processes respecti...
Text Solution
|
- Assume that C(6)H(6) and C(6)H(5)CH(3) form an ideal solution then Del...
Text Solution
|
- One mole of Ideal monatomic gas taken through Isochoric heating for 10...
Text Solution
|
- Work (W) is the path function. Which of the following order is incorre...
Text Solution
|
- Standar entropies of x(2), y(2) and xy(3) are 60,40 and 50JK^(-1) mol^...
Text Solution
|
- Temperature of an ideal gas increases in:
Text Solution
|
- As a rubber band is stretched, it gets warmer, when released, it gets ...
Text Solution
|
- A system undergoes two cyclic process 1 and 2. Process 1 is reversible...
Text Solution
|
- A certain gas is expanded from (1L, 10atm) to (4L, 5atm) against a con...
Text Solution
|
- The standard enthalpies of formation of CO(2) gas and HCOOH((l)) are -...
Text Solution
|
- For this process (overall change) which is correct
Text Solution
|
- P and Q are two arbitrarily chosen intensive variables then
Text Solution
|
- Assume that C(6)H(6) and C(6)H(5)CH(3) form an ideal solution then Del...
Text Solution
|
- A flask of 1L having NH(3)(g) at 2.0atm and 200K is connected with ano...
Text Solution
|
- A flask of 1L having NH(3)(g) at 2.0atm and 200K is connected with ano...
Text Solution
|
- pK for the hydrolysis of ATP is
Text Solution
|