Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-IONIC EQUILLIBRIUM-ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET ( ADVANCED))
- A solution contains 0.05M of each of NaCl and Na(2),CrO(4),. Solid AgN...
Text Solution
|
- For a general reaction given below, the value of solubility product ca...
Text Solution
|
- For a general reaction given below, the value of solubility product ca...
Text Solution
|
- For a general reaction given below, the value of solubility product ca...
Text Solution
|
- A certain weak acid has K(a), = 10^(-5). If the equilibrium constant f...
Text Solution
|
- A solution is saturated in SrCO(3), and SrF(2). The CO(3)^(2-) was fou...
Text Solution
|
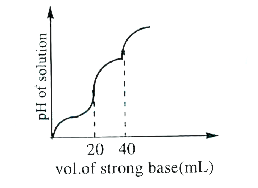
- 10 mLof H,A (weak diprotic acid) solutions is titrated against 0.1M Na...
Text Solution
|
- In a titration to 5OmL of 0.2M acetic acid (K(a), = 1.8 xx10^(-5) ), 0...
Text Solution
|
- Find pH of solution prepared by mixing 100ml, 0.1M Na(3),PO(4), and 20...
Text Solution
|
- pH of aqucous solution of 0.1M, NH(4),CI is found to be 5. The equilib...
Text Solution
|
- A solution of 0.01M Cd^(2+) contains 0.01IM NH(4),OH. What conc. of NH...
Text Solution
|